Professor Zeng Xun of Jinan Laboratory, alongside Professors Yang Yida and Yao Hangping from the State Key Laboratory of Diagnosis and Treatment of Infectious Diseases at the First Affiliated Hospital of Zhejiang University School of Medicine, have achieved a major breakthrough in vaccine immunology. Their pioneering work establishes Th1 cells as a key biological indicator for predicting individual variations following COVID-19 inactivated vaccine administration. This discovery challenges the traditional reliance on age to forecast vaccine efficacy, charting a new course for precision vaccination strategies grounded in immunological biomarkers. This research offers fresh insights into the immunological mechanisms underpinning vaccine efficacy. It also holds significant implications for countering viral evolution and guiding future vaccination programmes. The findings were published in November 2025 in the journal Advanced Science under the title ‘Pre-Existing Th1 Immunity Outperforms Age in Predicting Antibody Responses to SARS-CoV-2 Inactivated Vaccines’.

The elderly constitute a high-risk group for severe COVID-19, making it critically important to understand and enhance their immune response to COVID-19 vaccines. While considerable attention has been paid to factors contributing to inconsistent vaccine responses, host-related factors are often overlooked. Age is a recognised key determinant of vaccine response, yet studies elucidating vaccination outcomes in the elderly present particular challenges. This is largely due to the high heterogeneity of immune responses observed with advancing age and the prevalence of numerous underlying comorbidities in ageing populations. Consequently, developing novel biomarkers capable of sensitively and reliably predicting final immune protection levels prior to vaccination holds critical scientific and clinical value for optimising immunisation strategies and enabling personalised preventive interventions in elderly individuals.
Researchers established a model using aged C57 mice, dividing them into elderly (16–24 months) and young (6–8 weeks) cohorts. These mice received COVID-19 inactivated vaccines at D0 and D21. Subsequent blood and tissue sampling at various time points enabled detection of S1-specific antibodies and corresponding immunological markers. Surprisingly, the researchers observed significantly higher serum S1-specific antibody levels in aged mice post-vaccination compared to young mice (p<0.001), a finding rarely reported in prior studies. Comprehensive analysis of innate and adaptive immune cells revealed that the enhanced antibody response was closely associated with Th1 cell accumulation. Ageing mice exhibited Th1-polarised CD4+ T cells, with this Th1-polarised CD4+ T cell population displaying unique transcriptomic characteristics. The antibody type predominantly generated following COVID-19 inactivated vaccine immunisation was IgG2c, with the induced immune response exhibiting a Th1-biased character. Single-cell sequencing revealed that Th1 cells in aged mice exhibited a more robustly activated state, with significant upregulation of pathways including cellular antigen processing and presentation, T cell receptor signalling, JAK-STAT signalling, and chemokine signalling, alongside downregulation of pathways such as apoptosis. Following vaccination, these age-related transcriptional differences were markedly attenuated, particularly in Th1 cells. Post-vaccination Th1 cells in aged mice showed no discernible enrichment pathways distinct from those in young mice. The phenomenon of ‘age-related differential erasure’ observed in Th1 cells following COVID-19 inactivated vaccine administration represents a landmark discovery in the field of immune senescence research. While conventional wisdom posits immune senescence as cumulative irreversible damage, this finding demonstrates that the essence of aged Th1 cells lies in epigenetic silencing rather than permanent functional impairment. Strong antigenic stimulation can transiently release chromatin constraints, providing direct evidence for reversing immune senescence.
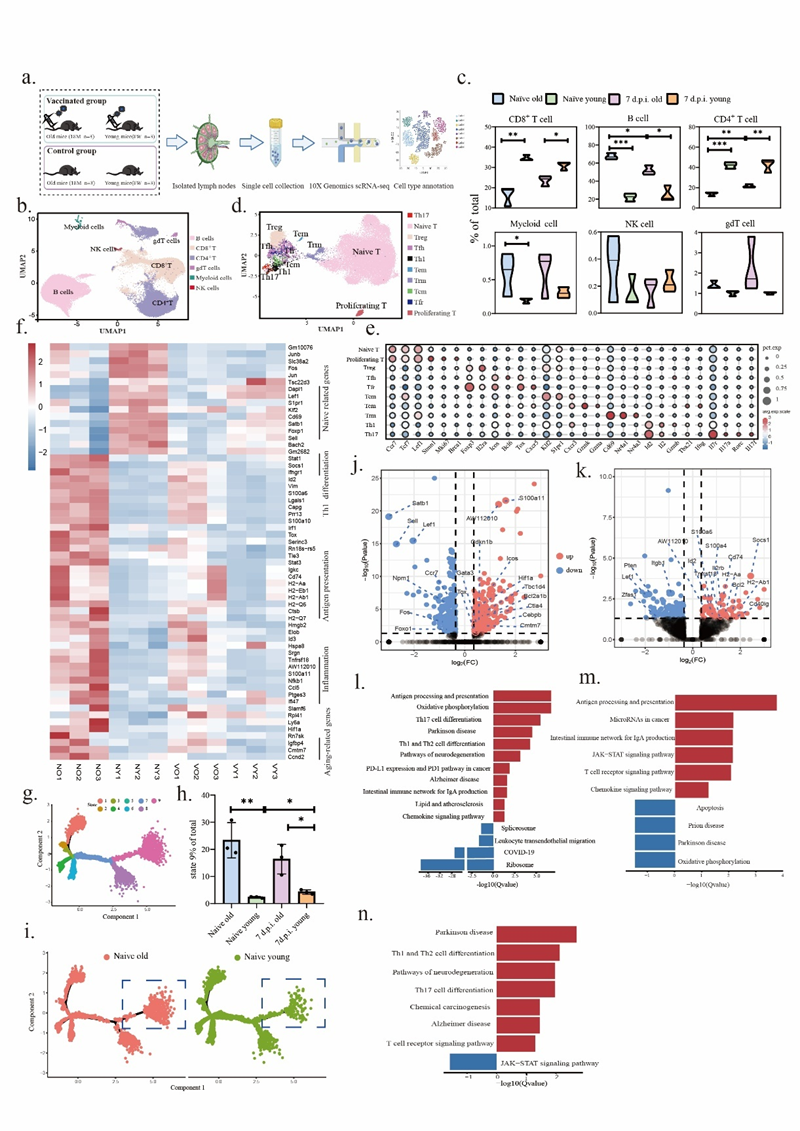
Figure 1. a Schematic workflow. b. Cluster analysis revealing immune cell subpopulations in draining lymph nodes. c. Subpopulation statistics. d. Identification of CD4+ T cell subsets. e. Bubble plot displaying marker genes for each CD4+ T cell subset. f. Heatmap of differentially expressed genes in CD4+ T cells between aged and young mice at baseline and post-vaccination day 14. g. CD4+ T cell differentiation trajectory diagram, with different colours representing distinct differentiation states. h. Proportion of state 9 cells in aged and young mice. i. Distribution of state 9 cells in baseline CD4+ T cells from aged and young mice. J-k. Volcano plots of differentially expressed genes in Tfh and Th1 cells between aged and young mice at baseline. l-m. KEGG enrichment analysis revealing differentially enriched pathways in Tfh and Th1 cells between aged and young mice at baseline. n. KEGG enrichment analysis revealing differentially enriched pathways in Tfh cells between aged and young mice at D14 post-inactivated COVID-19 vaccination.
Within human cohorts, immune cell composition varies considerably between elderly individuals. Specific antibodies induced by COVID-19 inactivated vaccines show no significant difference compared to younger individuals. Thus, human immune status is influenced by multiple factors, rendering age-based differentiation of vaccine protective efficacy highly biased. Stratified analysis by antibody concentration revealed that subjects with high antibody responses exhibited elevated baseline Th1 cell levels, which strongly correlated positively with post-vaccination antibody production. This indicates that T-cell background immunity plays a previously underestimated central role in shaping subsequent antibody responses. This challenges the traditional paradigm of predicting vaccine efficacy solely by age, highlighting the potential for personalised immunostrategies based on immunobiological markers. The researchers propose that baseline Th1 cells hold promise as a novel predictor of antibody generation following inactivated COVID-19 vaccination, surpassing age as a predictive factor. These findings reveal novel mechanisms of Th1 cells in vaccine-induced immune responses, offering fresh insights for developing more effective protective vaccines.
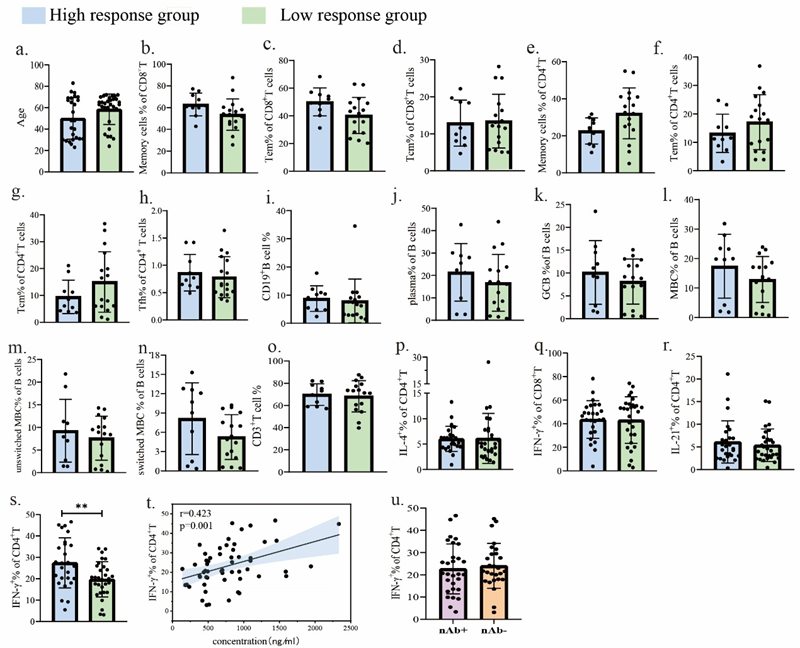
Figure 2. a. Age comparison between high- and low-antibody-response cohorts. b–r. Baseline distribution of CD8+ memory cells, CD8+ TEM cells, CD8+ TCM cells, CD4+ memory cells, CD4+ TEM cells, CD4+ TCM cells, Tfh cells, B cells, plasma cells, GCB, MBC, non-class-switched MBC, class-switched MBC, T cells, CD4+IL-4+, CD8+IFN-γ+, and CD4+IL-21+ at baseline. S. Comparison of Th1 levels between high and low antibody response groups at baseline. t. Correlation between baseline Th1 cells and S1-specific antibody levels following inactivated COVID-19 vaccination. u. Comparison of Th1 cells at baseline between neutralising antibody-positive and neutralising antibody-negative groups following COVID-19 inactivated vaccine administration.
Research Implications
This study systematically elucidates the differential immune response characteristics observed in aged individuals following COVID-19 inactivated vaccine administration by establishing an aged mouse model combined with single-cell transcriptomic analysis. It comprehensively maps the dynamic evolution of adaptive and innate immune cells during the ageing process. This work provides crucial foundational data and theoretical support for deepening our understanding of how immune ageing influences vaccine efficacy. It pioneers the proposal that Th1 cells serve as a key biological indicator for predicting individual variability following COVID-19 inactivated vaccine administration. This breakthrough challenges the traditional reliance on age for vaccine efficacy prediction, charting a new course for precision vaccination strategies grounded in immunological biomarkers.