Recently, the team led by Academician Li Lanjuan and Principal Investigator Professor Diao Hongyan at the Jinan Laboratory focused on the pathogenesis of inflammatory diseases. They systematically analysed the physicochemical properties of hydrogels, summarised four primary therapeutic mechanisms of hydrogels in such diseases, and conducted an in-depth analysis of their mode of action and functional positioning across different therapies. Furthermore, the team systematically explored current challenges and potential solutions for different polymer compositions and therapeutic approaches. This review, titled ‘Multifunctional hydrogels: Therapeutic strategies and advances in inflammation’, was published online in the European Polymer Journal.

Chronic inflammatory diseases, such as rheumatoid arthritis, inflammatory bowel disease, diabetes, Alzheimer's disease, cardiovascular disease, and cancer, are all closely associated with persistent inflammatory responses. Traditional drug therapies face limitations in efficacy due to issues such as high drug hydrophobicity, rapid metabolism, short half-lives, and significant side effects with long-term use. Hydrogels, formed as three-dimensional networks through the cross-linking of natural or synthetic macromolecules, serve as intelligent platforms for diverse therapeutic approaches. This is due to their highly tunable three-dimensional network architecture, inherent bioresponsiveness, and capacity for integrating multifunctional modules.
Drawing upon hydrogels' physicochemical properties, this review dissects four primary therapeutic mechanisms: targeted drug delivery, regenerative tissue scaffolds, immunomodulation, and multifunctional anti-inflammatory dressings. It categorises hydrogel-mediated therapeutic strategies and their underlying mechanisms. 1. As targeted drug delivery systems, they construct multidimensional therapeutic platforms through physical protection, environmental responsiveness, and spatiotemporal control, enhancing drug stability and precision; 2. As tissue regeneration scaffolds, they integrate functions including stem cell delivery, barrier protection, structural mimicry, and bioactive modification, advancing from ‘immunomodulation’ towards ‘precise tissue regeneration’ to achieve dual anti-inflammatory and regenerative effects; 3. For immunomodulation, they incorporate bioactive molecules, functional peptides, natural polysaccharides, and nanomaterials to develop smart responsive hydrogels that regulate the immune microenvironment; 4. As multifunctional anti-inflammatory dressings, they not only provide physical barriers and moist environments but also integrate antimicrobial, antioxidant, and immunomodulatory functions, offering comprehensive therapeutic solutions for chronic wounds and infections.
The design and development of anti-inflammatory hydrogels have been propelled by cumulative research on functional hydrogels. A comprehensive understanding of hydrogel-mediated anti-inflammatory pathways and their molecular mechanisms will facilitate significant advancements in treating inflammatory diseases. This review outlines recent progress in hydrogel-based strategies for inflammation management, encompassing diverse mechanisms of action. The emergence of hydrogels addresses limitations encountered in conventional inflammatory treatment models, offering novel therapeutic approaches for inflammatory conditions.
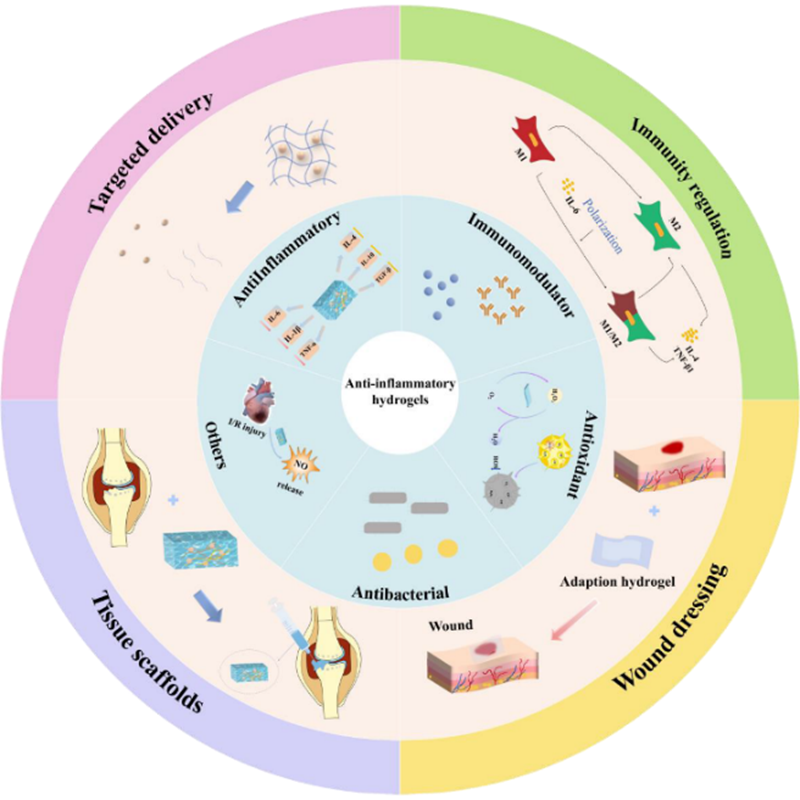
Scientific Insight: The therapeutic efficacy of hydrogels stems from precise, designable interactions between their physicochemical properties and inflammatory processes. Beyond serving as drug carriers, hydrogel components, modulus, pore size, adhesion, and responsiveness directly intervene in and remodel the physical, chemical, and biological aspects of inflammation. As a frontier direction in inflammatory disease therapy, hydrogel technology's multi-mechanism synergistic and customisable design characteristics open new possibilities for future precision medicine.
This research received funding from the Shandong Provincial Key R&D Programme (SYS202202), the National Key R&D Programme (2021YFA1301100, 2021YFA1301101), the National Natural Science Foundation of China (8240022434), and the Jinan Microecological Biomedicine Shandong Laboratory Science (JNL-2022001A, JNL-2023009Q, JNL-2023007Q).
Original link: https://doi.org/10.1016/j.eurpolymj.2025.114064