The research team led by Professor Wang Hangxiang at Jinan Laboratory has achieved a significant breakthrough in the field of tumor immunotherapy. This study not only precisely reveals the key mechanism limiting the clinical application of STING agonists but also successfully constructs "Immunomodulatory binary nanoparticles" through structure-guided rational drug design and precise nano-assembly technology. This innovative formulation can actively target and remodel the tumor immune microenvironment, effectively reversing the immune tolerance mediated by overactivation of the STING pathway. This formulation achieves, for the first time, intravenous delivery of STING agonists, breaking the clinical limitation that such drugs require intratumoral injection. Simultaneously, it provides a novel technical approach to address the adaptive immune evasion triggered by STING therapy. These findings were published online on October 23, 2025, in the Journal of Clinical Investigation under the title "Targeting STING–induced immune evasion with nanoparticulate binary pharmacology improves tumor control in mice."
Stimulator of interferon genes agonist therapy is one of the most promising strategies in tumor immunotherapy. The STING pathway can activate the body's innate immune system, converting immunologically "cold tumors" into "hot tumors," thereby allowing immune cells to infiltrate and kill cancer cells. Although STING agonists demonstrate excellent anti-tumor activity in preclinical studies, their clinical efficacy has been unsatisfactory. The mechanisms of resistance and immune evasion associated with STING agonist drugs are urgent problems that need to be solved.
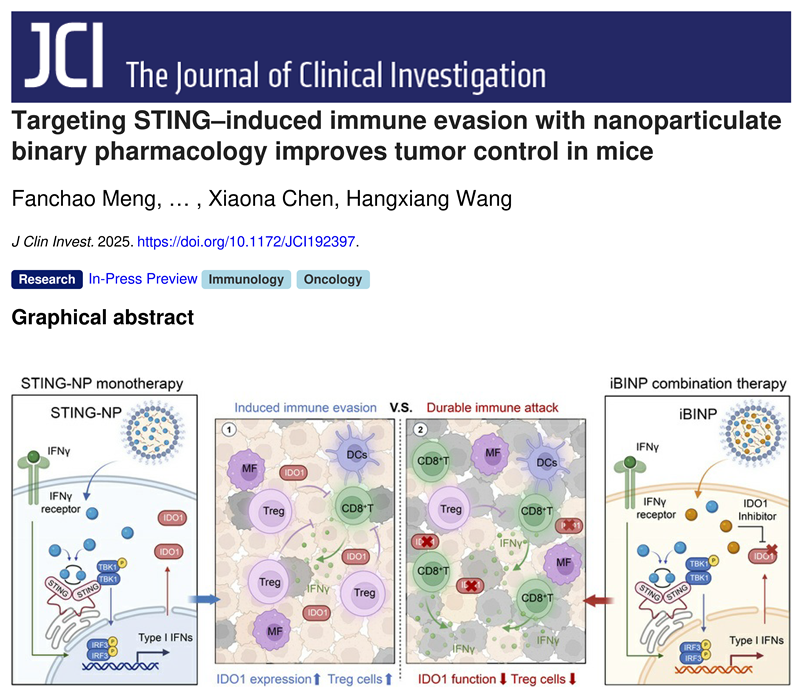
This study first addressed a key scientific question: Why is the efficacy of STING agonists self-limiting? Through multi-omics analysis and a series of mechanistic validations, the team discovered that while activating the type I interferon pathway, STING agonists also induce a negative feedback loop that promotes immune tolerance and evasion. Specifically, the IFN-γ induced by STING agonists leads to the high expression and functional upregulation of a key immune metabolic checkpoint in the tumor microenvironment–indoleamine 2,3-dioxygenase 1. The IDO1 metabolic enzyme creates an immunosuppressive tumor microenvironment by regulating the tryptophan/kynurenine metabolic pathway, promoting the differentiation of regulatory T cells, thereby suppressing the function of effector T cells and inducing their apoptosis, ultimately leading to immune evasion during STING agonist treatment. To confirm that IDO1 is the key switch mediating resistance to STING agonists, the research team constructed Ido1 knockout and Ido1 overexpressing tumor models. Experimental results showed that in the Ido1 overexpressing tumor models, the efficacy of the STING drug was significantly weakened; whereas in the Ido1 knockout tumor models, the activity of the single STING agonist was comparable to that of iBINP. This key experiment demonstrated that upregulation of IDO1 expression is the core mechanism mediating resistance to STING agonists.
Aiming at this negative feedback mechanism, the research team designed and constructed a "binary prodrug co-assembly" strategy. They performed lipidization modifications on two small molecule compounds with distinct physicochemical properties, poor solubility, and unsuitability for intravenous delivery – a STING agonist and an IDO1 inhibitor. This structural reconstruction at the molecular level enables the two different prodrugs to co-assemble via supramolecular non-covalent interactions, forming a novel immunomodulatory nanomedicine that can be administered by intravenous infusion and exhibits long circulation in the blood. The ingenuity of this delivery system lies not in simple drug combination, but in efficiently addressing the critical challenge of mutual synergy between different drugs through clever medicinal chemistry and innovative formulation strategies. More crucially, iBINP ensures that different drug molecules can achieve spatiotemporal co-delivery at the tumor site and draining lymph nodes, which is a prerequisite for their synergistic action.
This iBINP drug system demonstrated favorable activity in multiple refractory tumor models. In subcutaneous colorectal cancer and breast cancer models, the therapeutic window and efficacy of iBINP were significantly superior to single-agent treatments or the combination of free drugs. More interestingly, in an acquired resistance tumor model for STING agonists established by the team, STING agonist monotherapy had lost its tumor-suppressive effect, but iBINP could efficiently inhibit tumor growth and significantly prolong survival, successfully alleviating the tumor immune evasion and tolerance induced by the STING drug. Furthermore, this strategy also showed excellent therapeutic potential in a refractory colitis-associated cancer model and a postoperative lung metastasis model.
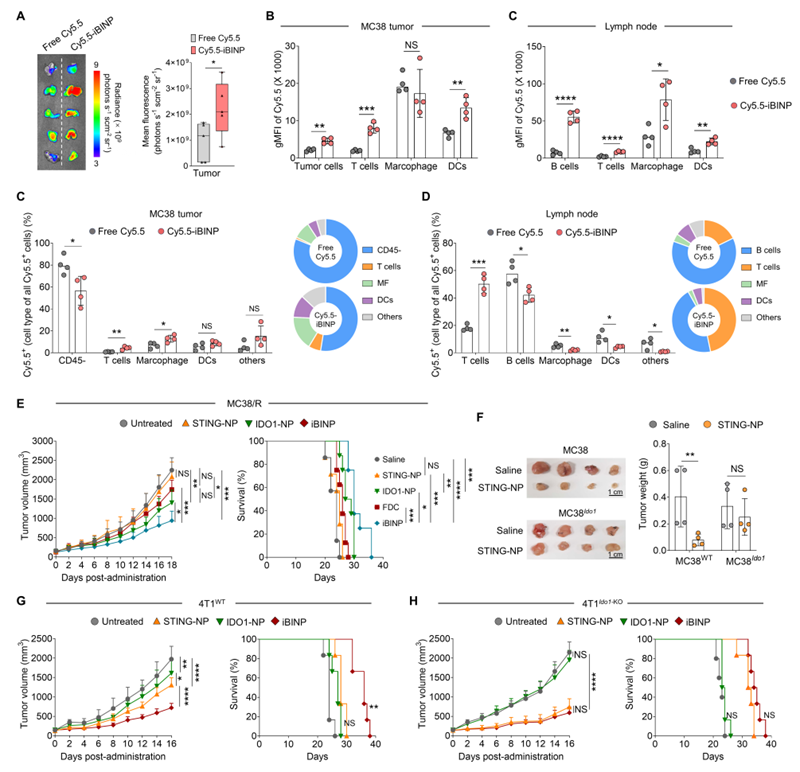
Figure 1. In vivo biodistribution of iBINP and validation of its anti-tumor mechanism.(A) Fluorescence imaging of ex vivo tumor tissues after intravenous injection of Cy5.5-labeled iBINP or free Cy5.5 dye.(B, C) Flow cytometry analysis comparing the uptake of iBINP by various cell types in MC38 tumors and tumor-draining lymph nodes.(D) Distribution and percentage analysis of Cy5.5-labeled iBINP among different cell types in MC38 tumors and tumor-draining lymph nodes.(E) Tumor growth curves and survival analysis of mice in various treatment groups in the STING agonist acquired resistance subcutaneous tumor model.(F) Comparison and statistics of ex vivo tumors from MC38 wild-type tumors and Ido1 overexpressing MC38 tumors treated with STING agonist.(G, H) Tumor growth curves and survival of mice in various treatment groups in wild-type 4T1 and Ido1 knockout 4T1 subcutaneous tumor models.
In summary, this study not only reveals the resistance mechanism of STING drugs against tumors but also provides a novel nanomedicine strategy based on synthetic chemistry and supramolecular chemistry to address this negative feedback loop. iBINP degrades in vivo, generating only the two drugs and docosahexaenoic acid, a polyunsaturated fatty acid very important for the human body. This research offers a new solution for the clinical translation of STING agonists, holding high clinical translational value.
Research Inspiration:This work innovatively proposes a "mechanism-guided co-delivery" design concept. By analyzing the negative feedback loop during drug action, it specifically constructs nanomedicines with spatiotemporal synergistic effects, providing a new paradigm for overcoming resistance in tumor immunotherapy.
Professor Wang Hangxiang's research group has long been engaged in basic and clinical translational research on anti-tumor immune drugs and delivery systems. In recent years, they have published over 100 papers in academic journals such as PNAS, Cancer Research, Nature Communications, Advanced Materials, ACS Nano, Biomaterials, and Journal of Controlled Release. Some innovative drug achievements have been transferred and entered the clinical translation stage. This research was funded by the Jinan Microecological Biomedicine Shandong Laboratory Project, the Major Basic Research Project of Shandong Provincial Natural Science Foundation, and the General Program of the National Natural Science Foundation of China.