Recently, Professor Wang Hangxiang's team at the Jinan Microecological Biomedicine Shandong Laboratory addressed the challenge of low response rates to immune checkpoint inhibitors in melanoma. Building upon the mechanism of mitochondrial-targeted induction of immunogenic cell death (ICD), they developed a novel strategy involving a mitochondrial-targeted ‘pseudo-stealthy’ nanophotosensitiser. This approach significantly enhances immunotherapy efficacy by efficiently disrupting tumour cell mitochondria and activating systemic anti-tumour immunity. The study, titled ‘Mitochondria-targeting pseudo-stealthy nanophotosensitizer as a potent immunogenic cell death inducer to unleash the cancer-immunity cycle for melanoma therapy’, was published in the journal Acta Biomaterialia.
Melanoma, originating from melanocytes, ranks among the most aggressive cancers. Whilst immune checkpoint inhibitors (such as anti-PD-1/PD-L1) have become pivotal in melanoma treatment, approximately 50% of patients fail to benefit due to insufficient T-cell infiltration and immune-suppressive factor accumulation within the tumour microenvironment. Mitochondria, serving as cellular powerhouses, also function as critical hubs regulating immune responses. Targeting mitochondria induces tumour cells to release substantial damage-associated molecular patterns (DAMPs), triggering potent immunogenic cell death (ICD) that activates dendritic cell (DC) maturation and T-cell anti-tumour responses. While conventional photodynamic therapy (PDT) can induce ICD, it suffers from poor photosensitiser targeting, rapid clearance, and inadequate tumour penetration.
The research team innovatively designed the Mito-PM micellar nanoplatform. By sparsely modifying the micelle surface with triphenylphosphine (TPP) cations, they ensured prolonged circulation retention and high tumour accumulation while enabling efficient enrichment within tumour cell mitochondria via the TPP-mediated mitochondrial membrane potential gradient. Upon near-infrared light (660 nm) irradiation, it generates substantial reactive oxygen species (ROS), directly disrupting mitochondrial structure and inducing potent ICD. Cellular experiments confirmed Mito-PM's specific targeting of cellular mitochondria, causing mitochondrial dysfunction and inducing cellular ICD. In the B16F10 animal model, Mito-PM combined with anti-PD-1 antibody demonstrated significant synergistic therapeutic effects. Results showed that tumour volume in the Mito-PM group was significantly smaller than in the non-mitochondrial-targeted phototherapy group, while combination therapy with anti-PD-1 antibody achieved optimal tumour suppression, with tumour volume on day 15 significantly reduced compared to the anti-PD-1 monotherapy group. Pathological analysis revealed pronounced tumour cell apoptosis, proliferation inhibition, and immune cell infiltration in the combination therapy group, alongside marked activation of ICD.
These findings demonstrate that reducing the surface positive charge density of nanomicelles without compromising mitochondrial accumulation effectively improves their pharmacokinetic properties. Mitochondria-targeted photodynamic therapy not only enhances anti-tumour immune responses and therapeutic efficacy but also synergises with immune checkpoint inhibitors to produce superior antitumour outcomes.
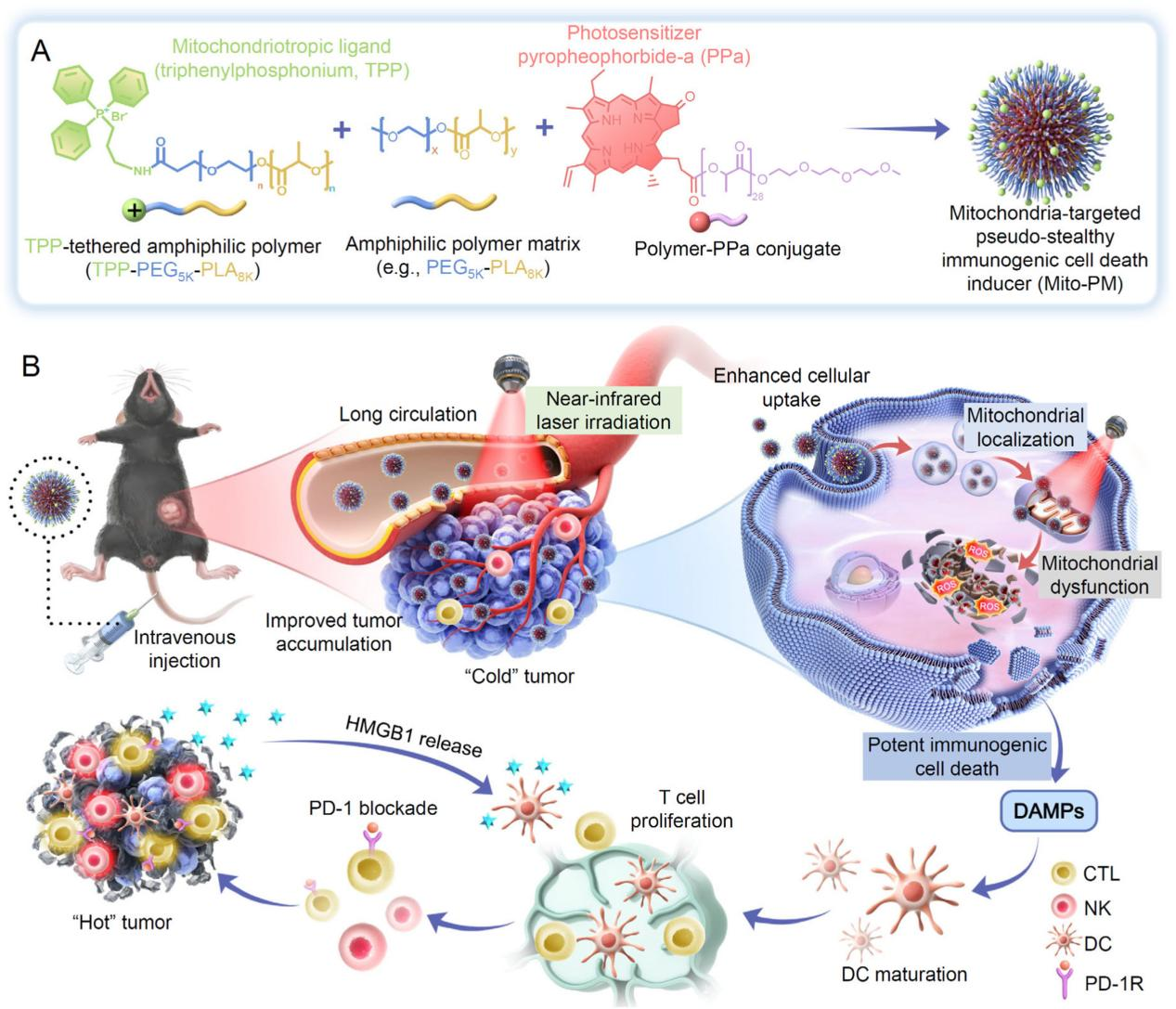
Scientific Implications: This study innovatively proposes a ‘pseudo-stealth’ nanodesign concept featuring low-density cationic modification, offering novel insights to address the dual challenges of poor photosensitiser targeting and weak immune activation. It paves new pathways for combined immunotherapy in melanoma treatment.
This research was supported by the Major Basic Research Programme of the Shandong Natural Science Foundation (ZR2023ZD59) and the Jinan Microecological Biomedicine Shandong Laboratory Science (JNL-2025007B).
Original article link: https://www.sciencedirect.com/science/article/pii/S174270612500532X