Recently, the research team led by Academician Gu Xiaosong, Professor Jiang Chunping (Deputy Director of the Laboratory), and Principal Investigator Professor Wu Junhua at the Jinan Laboratory conducted in-depth research on the efficacy of oncolytic viruses in controlling postoperative tumour recurrence through in situ extended activation of anti-tumour immunity initiated during surgery. This study, titled ‘In situ extended immune activation instantly after tumour resection by oncolytic virus controls postoperative tumour recurrence’, was published in the journal Cell Reports Medicine.

Cancer represents a major disease threatening human health and life. For patients eligible for surgery, tumour resection is often the preferred treatment approach. However, postoperative recurrence remains one of the most significant challenges requiring resolution. The wound healing process and associated inflammation can also contribute to tumour recurrence following surgery. Currently, chemotherapy and radiotherapy are commonly employed clinically to control postoperative recurrence, but these methods often carry substantial toxic side effects in certain circumstances. Consequently, there is an urgent need for safe and effective therapeutic strategies to manage tumour recurrence after surgery.
The causes of tumour recurrence after surgery are primarily attributed to local tumour micro-invasion and circulating tumour cells. These not only suppress innate immune responses in multiple ways but also facilitate the escape of tumour cells from infiltrating immune effector cells. Furthermore, the wound healing process and associated inflammation can induce an immunosuppressive microenvironment in the surgical local area, followed by systemic immunosuppression. This aids tumour cells in achieving immune escape and lying dormant until recurrence. Cancer immunotherapy has advanced rapidly in recent years. Among these approaches or agents, some eliminate tumour cell immune evasion and directly induce anti-tumour immunity; certain methods hold potential value in controlling postoperative recurrence and metastasis. Consequently, tumour immunotherapy should possess significant potential application value in preventing postoperative recurrence.
Systemic immunotherapies, such as immune checkpoint inhibitors, CAR-T, or CAR-NK cell therapies, primarily aim to achieve long-term, effective systemic immune surveillance. Research indicates that intratumoral immunotherapy is safer than systemic treatment and sometimes more effective. Localised, concentrated treatment at the tumour site can break local immune tolerance, induce systemic anti-tumour immunity, and simultaneously avoid severe side effects. Consequently, in situ immune activation represents a highly promising strategy for tumour immunotherapy and preventing postoperative recurrence. Another critical point is that wound healing and inflammation commence immediately upon tumour excision. Therefore, to effectively control postoperative recurrence, immune activation should be induced as early as possible. Furthermore, postoperative wound healing is not a short-term process. Thus, the most ideal approach would be to implement in situ immune modulation immediately postoperatively and maintain it for a sustained period.
Oncolytic virotherapy represents a highly promising branch of cancer immunotherapy, utilising viruses with antitumour properties that selectively replicate within and lyse tumour tissue without harming normal structures. Beyond their direct oncolytic effect on tumour cells, these viruses can induce systemic antitumour immune responses, thereby transforming ‘cold’ tumours into ‘hot’ tumours. This outcome enhances tumour sensitivity to other therapeutic modalities, thereby creating opportunities for synergistic anti-tumour strategies. Among all oncolytic virus types, adenovirus, herpes simplex virus, and vaccinia virus represent the three most extensively studied oncolytic viruses in clinical trials over the past decade, indicating their potential for future applications.
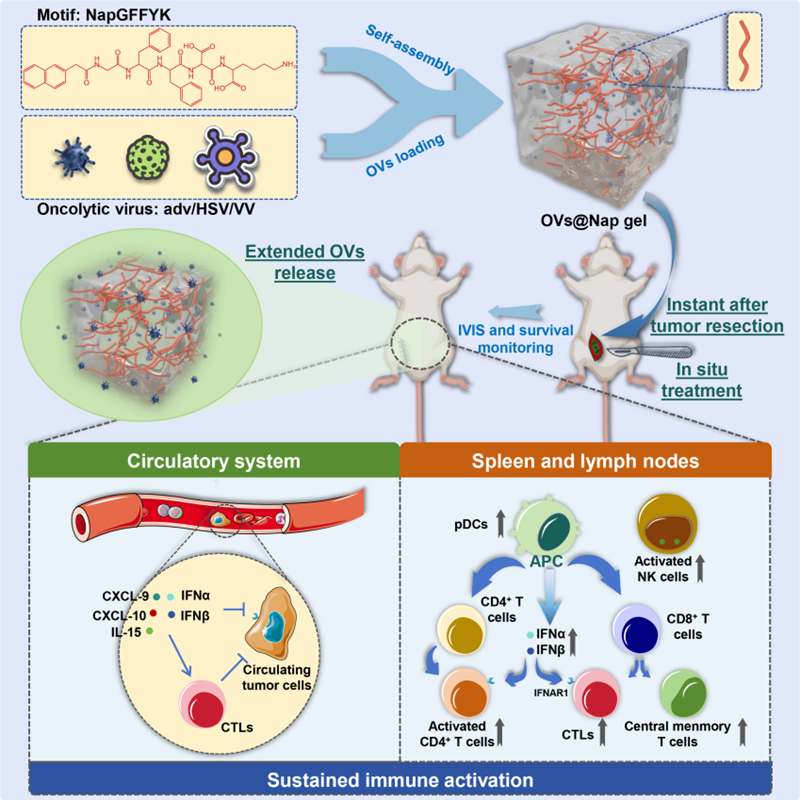
In this study, the team achieved sustained intraoperative release of oncolytic viruses (adenovirus adv, herpes simplex virus HSV, or vaccinia virus VV) at the surgical site. In an in situ mouse model of breast cancer, immediate intraoperative implantation of adv@Nap gel following tumour excision sustained anti-tumour immune activation, controlling postoperative tumour recurrence. Furthermore, the team extended this concept to other viral vectors (including HSV and VV), validating the findings in humanised mouse tumour models. These discoveries provide crucial therapeutic strategies and theoretical foundations for developing methods to control tumour recurrence post-surgery.
The team first assessed the immune-activating capacity of type V oncolytic adenovirus (adv). In the 4T1 breast cancer model, a single intratumoral injection significantly increased CD8+ T cells, cytotoxic CD8+ T cells (IFN-γ+/GZMB+), and central memory CD8+ T cells within both tumours and spleens, establishing an immunological foundation for subsequent prevention of postoperative recurrence.
To address the impracticality of in situ oncolytic virus therapy after tumour tissue removal, the team employed a self-assembling peptide hydrogel system (NapGFFYK hydrogel) capable of local drug delivery in vivo. This system ensures sustained in situ release of the oncolytic virus through gradual degradation of the hydrogel matrix over time. The mechanical stability of adv@Nap gel is crucial for its application as a viral vector. Rheological characterisation confirmed adv@Nap gel maintains sustained mechanical stability both in vitro and in vivo. PCR and TCID50 assays demonstrated consistent in vitro and in vivo release of adv while preserving viral infectivity.
In a 4T1 breast cancer recurrence model, intraoperative in situ placement of adv@Nap gel significantly suppressed tumour recurrence and metastasis, prolonging mouse survival. Delaying administration by 7 days or switching to subcutaneous/intravenous routes markedly diminished efficacy, underscoring the critical importance of ‘intraoperative immediate + in situ’ delivery for controlling postoperative tumour recurrence.
Adv continuously released from adv@Nap gel rapidly activates innate immunity, including pDCs, inducing the type I interferon (IFN-α/β) pathway; Subsequently, sustained expansion of CD4+/CD8+ T cells, central memory T cells, and antigen-specific cytotoxic T cells occurs, with immune memory persisting for approximately 4 weeks and conferring resistance to 4T1 re-challenge.
HSV@Nap gel or VV@Nap gel similarly significantly suppressed postoperative tumour recurrence. Consistent findings in the B16F10 melanoma model suggest this delivery platform is applicable to multiple oncolytic viruses.
In the humanised triple-negative breast cancer model derived from MDA-MB-231 cells, adv@Nap gel markedly enhanced human CD8+ T-cell activation and IFN-α/β levels, prolonging survival without significant toxicity. This provides robust evidence for subsequent clinical translation.
Research Implications: The timeliness of immunotherapy and sustained tumour-specific immune activation at the surgical site significantly influence postoperative prognosis. This study provides theoretical support for clinical immunotherapy applications in managing recurrence, offering a crucial therapeutic option for controlling postoperative relapse.
This research received funding from the Shandong Natural Science Foundation (Grant No. ZR2025MS1306), Shandong Provincial Laboratory Project (Grant No. SYS202202), National Natural Science Foundation of China (Grant Nos. 81972888, 82272819), and Jinan Microecological Biomedicine Shandong Laboratory Science Research Projects (Grant Nos. JNL-2025008B, JNL-2025009B, JNL-2025011B, JNL-2025010B, JNL-2025012B, JNL-2023017D), and the Provincial Key R&D Programme (No. BE2022840).
Original link: https://doi.org/10.1016/j.xcrm.2025.102399