Recently, the research team led by Academician Gu Xiaosong, Professor Jiang Chunping (Deputy Director of the Laboratory), and Principal Investigator Professor Wu Junhua at the Jinan Laboratory has systematically analysed the mechanisms by which tumour-associated macrophages (TAMs) adapt to the tumour microenvironment and mediate immune suppression through amino acid metabolic reprogramming. systematically dissected the mechanisms by which tumour-associated macrophages (TAMs) adapt to the tumour microenvironment and mediate immunosuppression through amino acid metabolic reprogramming. The review further explores strategies targeting relevant metabolic pathways to enhance anti-tumour immunotherapy. This review, titled ‘Dynamic remodelling of amino acid metabolism in tumour-associated macrophages: Fueling immunosuppression, reshaping tumour niches, and unlocking metabolic checkpoints’, was published in the Journal of Advanced Research (Impact Factor: 13.0, Q1 in CAS).
Metabolic reprogramming has become a hallmark of tumours. To adapt to microenvironments characterised by hypoxia, stress, unrestrained proliferation, and nutrient deprivation, tumour cells are compelled to increase lipid metabolism and glucose uptake while undergoing amino acid metabolic reprogramming to meet their demands. Abnormal amino acid metabolism in tumour cells—manifesting as heightened uptake and alterations in metabolic pathways or key enzymes—can lead to amino acid deficiency or accumulation of specific metabolites within the tumour microenvironment (TME), thereby suppressing anti-tumour immune responses. As the predominant immune cell population within tumours, tumour-associated macrophages (TAMs) also rely on amino acids to achieve functional adaptation to the TME.
To adapt to metabolic and signalling alterations within the TME, TAMs undergo metabolic reprogramming that confers immunosuppressive and pro-tumour functions. Within the TME, TAMs extensively utilise amino acid metabolism to promote their pro-tumour state and functional transformation. Polarised TAMs exhibit marked capacity for tyrosine and arginine uptake and metabolism, primarily displaying immunosuppressive phenotypic and functional characteristics. Moreover, amino acid metabolism within TAMs triggers the exchange of cytokines, metabolites, and signalling molecules with other cells, thereby reshaping the TME state. Targeting amino acid metabolism to convert TAMs' pro-tumour state into anti-tumour function represents a novel immunotherapeutic strategy with significant application potential. Consequently, how TAMs adapt to the TME by remodelling amino acid metabolism, and how their immunoregulatory networks influence tumour progression and therapeutic response, represent critical research questions in contemporary tumour immunology.
This review dissects how TAMs adapt to TME alterations and suppress anti-tumour immune responses through amino acid metabolic responses. It maps the immunometabolic crosstalk through which TAMs reshape immunity, highlighting nutrient competition and metabolic by-products as drivers of immune dysfunction. It evaluates pathways such as IFN-γ-JAK-STAT1 and IL-6/JAK2/STAT3 for their potential to reprogramme TAMs and reverse immunosuppression. Considering amino acid metabolism's impact on tumour therapy, this paper summarises and analyses the immunometabolic characteristics of amino acids in TAMs and their crosstalk with various aspects of the TME. It explores their potential as biomarkers for immune responses, suggesting that combining immunotherapy with metabolic interventions could better unlock the potential of anti-tumour treatments.
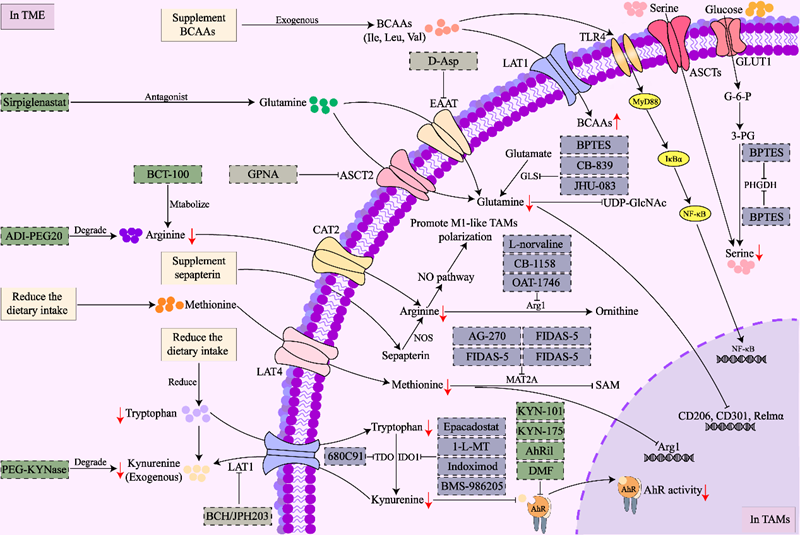
Research Implications: The presence and metabolism of amino acids such as tryptophan, arginine, glutamine, serine, branched-chain amino acids, methionine, and taurine play crucial roles in the heterogeneous polarisation of TAMs. This not only determines their anti-tumour or pro-tumour functional states but also significantly influences TAM interactions with other immune cells within the TME and their execution of immune evasion functions. Modulating TAM-associated amino acid metabolism may offer novel research avenues for metabolic checkpoints in tumour immunotherapy. Examples include Epacadostat, 1-L-MT, and Indoximod—drugs targeting the key tryptophan metabolic enzyme IDO1; and Ilantimod, KYN-101, and KYN-175—drugs targeting the sensor AhR.
This research was supported by the Shandong Provincial Key R&D Programme (SYS202202, SYS202502), the National Natural Science Foundation of China (82272819, 81972888), and the Jinan Microecological Biomedicine Shandong Laboratory Science (JNL-2025008B, JNL-2025009B, JNL-2025011B, JNL-2025010B, JNL-2023017D), and the Jiangsu Provincial Key R&D Programme (BE2022840).