Recently, the research team led by Professor Li Xingang, Deputy Director of the Laboratory, and Principal Investigator Zhang Di explored the effects of glioma-derived exosomes on astrocytes and their underlying mechanisms. They also investigated differences in glioma cell activation of astrocytes under hypoxic versus normoxic conditions. The findings were published in Cell Death & Disease under the title ‘Inhibition of hypoxic exosomal miR-423-3p decreases glioma progression by restricting autophagy in astrocytes’.

As the primary primary tumour of the central nervous system, glioma typically carries a poor prognosis for patients. Current treatment regimens employ a multimodal approach centred on surgical resection, supplemented by radiotherapy, chemotherapy, and molecular targeted interventions. However, existing therapies remain markedly inadequate in controlling tumour progression and preventing recurrence, largely due to tumour cell resistance and invasive growth characteristics.
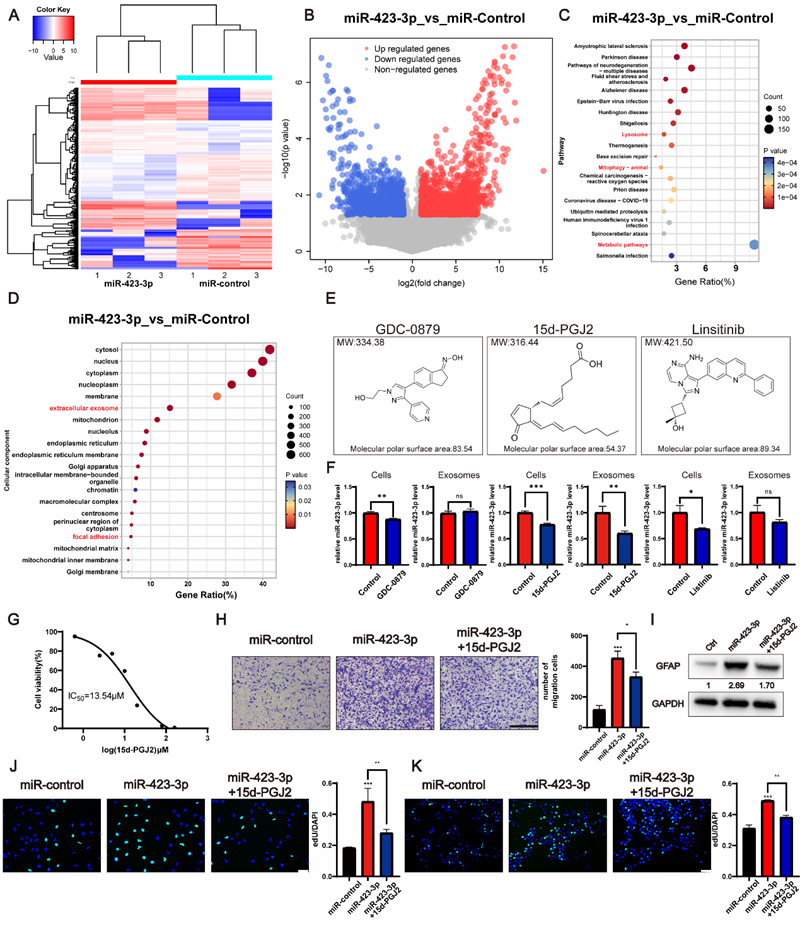
This study isolated glioma cell-derived exosomes under hypoxic and normoxic conditions, treated astrocytes with these exosomes, and subsequently employed transwell assays, edU, ELISA to investigate the activated astrocyte phenotype and differential activation markers. Western blot and transmission electron microscopy validated differences in autophagy levels within activated astrocytes, while miRNA array analysis identified key molecules within glioma exosomes inducing astrocyte activation. Transcriptome sequencing identified differentially expressed genes in activated astrocytes, and the cMAP database predicted potential drugs inhibiting astrocyte activation.
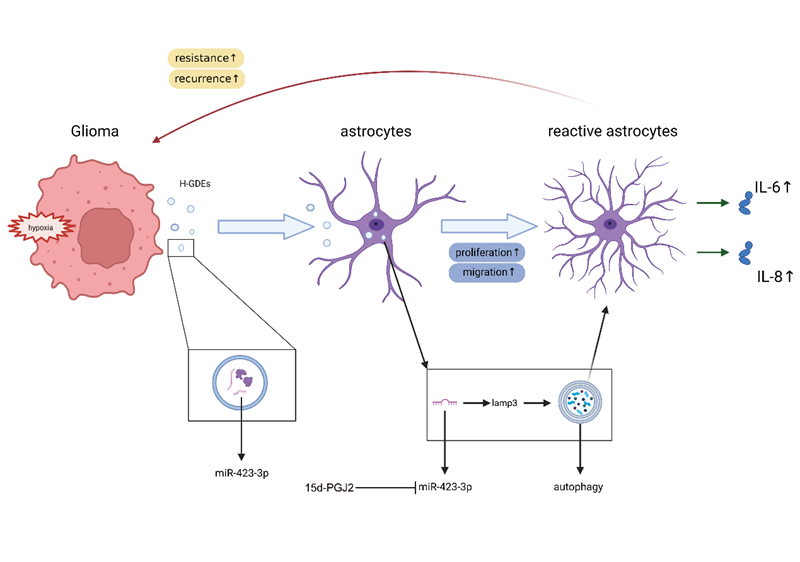
Compared to normoxic glioma exosomes, hypoxic glioma exosomes exhibited stronger astrocyte-activating effects, with mechanisms closely linked to cellular autophagy. The expression level of miR-423-3p in hypoxic exosomes was significantly higher than in normoxic exosomes. This microRNA was demonstrated to activate astrocytes by mediating autophagy. Mechanistic studies further revealed that 15d-PGJ2 effectively inhibits this astrocyte activation process by antagonising the biological activity of miR-423-3p, thereby identifying a potential therapeutic intervention target.
Scientific Implications
This study validates the existence of a positive feedback loop within the glioma microenvironment: hypoxic glioma exosomes induce astrocyte activation, and activated astrocytes further promote glioma progression. It elucidates specific operational mechanisms within the glioma tumour microenvironment and proposes 15d-PGJ2 as a potential therapeutic agent for glioma, offering novel insights for clinical glioma treatment.
This research was supported by the National Natural Science Foundation of China (82071512, 82203760, 81701329, 82102960), the Shandong Provincial Natural Science Foundation (ZR2019ZD33), the Shandong Provincial Laboratory Programme (SYS202202), and the Jinan Microecological Biomedicine Shandong Laboratory(JNL-2022003A).