Recently, Professor Cao Yi from the Regenerative Medicine and Tissue Engineering Microfabrication Platform at the Jinan Microecological Biomedicine Shandong Laboratory, in collaboration with the team led by Jiang Qing from the Drum Tower Hospital Affiliated to Nanjing University, addressed the application challenges in the field of stem cell transplantation for bone regeneration. Based on their foundational research on stem cell response to mechanical stimuli, they creatively employed a liquid-liquid phase separation soft template design strategy to develop a programmable spatiotemporal mechanical cue hydrogel with large pores. By regulating the mechanical microenvironment of stem cells, this hydrogel promotes bone tissue regeneration, offering a new strategy for stem cell-assisted bone defect repair. This study, titled ‘Hydrogels with programmed spatiotemporal mechanical cues for stem cell-assisted bone regeneration,’ was published in Nature Communications (IF: 15.7, a top journal in the first zone of the Chinese Academy of Sciences).

Bone defects are a common and severe clinical issue, particularly large or complex defects, which not only impair patients' quality of life but also lead to long-term functional disabilities, imposing a significant burden on individuals and society. Therefore, there is an urgent need for safe and efficient repair strategies to promote bone tissue regeneration. Stem cells, with their multi-potent differentiation potential and immune regulatory functions, are widely regarded as ideal seed cells for bone tissue engineering. However, the survival, homing, and differentiation efficiency of stem cells after transplantation remain limited by the microenvironmental support at the defect site. How to precisely regulate stem cell behaviour to enhance bone repair outcomes has become a core challenge in this field.
Bone tissue is a highly mechanically dependent structure, and its regeneration process requires not only appropriate biochemical signals but also reasonable mechanical stimuli to guide cell proliferation and differentiation. Numerous studies have shown that changes in material mechanical properties (including stiffness, elasticity, and degradation processes) directly influence stem cell fate decisions. An ideal bone repair scaffold should simultaneously satisfy mechanical support and tissue growth coordination at both temporal and spatial scales to achieve optimal regenerative outcomes.
To address this challenge, our research team developed a technical workflow based on macroporous hydrogel materials to decouple matrix elasticity from stem cell differentiation, and applied it in stem cell transplantation cases. This resolved the difficulty of simultaneously achieving cell proliferation and qualitative differentiation in bone tissue repair, This has improved the performance metrics of stem cell transplantation for bone repair, establishing a biomimetic macroporous gel-based hard tissue repair technology system. This provides a technical pathway for the further translational application of stem cell transplantation in tissue repair, combining material and mechanical coupling.
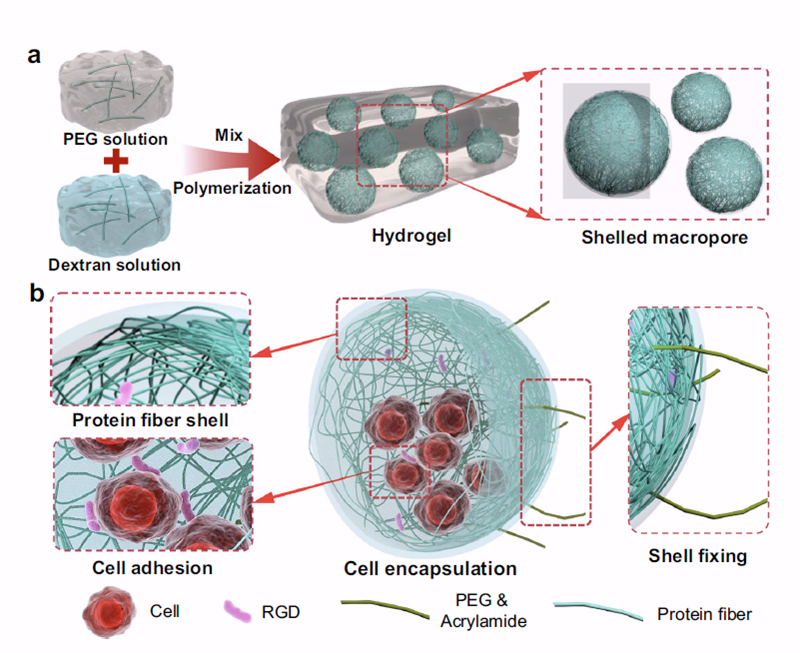
This hydrogel system consists of three core modules: first, a macroporous structure is constructed using a liquid–liquid phase separation strategy, providing channels for cell infiltration and tissue ingrowth; Second, a shell-hardened structure composed of protein nanofibres is formed on the pore walls, providing sustained mechanical support and stimulation; Finally, by adjusting the ratio of degradable and non-degradable polymer components, the stiffness and degradation process of the hydrogel are spatially and temporally controlled, enabling it to align with the rhythm of bone tissue formation.
In rabbit and pig models of large bone defects, this programmable mechanical hydrogel synergistically interacts with stem cells to significantly enhance the volume, thickness, and quality of new bone formation, whereas using hydrogel or stem cells alone cannot achieve the same repair effects. This strategy offers an efficient, controllable, and clinically translatable novel material solution for bone defect treatment.
Research Insights
This study established a large-pore hydrogel system with programmable spatiotemporal mechanical signals, enabling precise regulation of the mechanical microenvironment for stem cells and thereby efficiently promoting bone tissue regeneration. This strategy combining spatiotemporally controllable mechanical stimulation with tissue engineering scaffolds not only provides new insights for bone defect repair but also offers important references for designing regenerative materials applicable to other mechanically dependent tissues (such as cartilage and tendons).
Associate Professor Xue Bin from the Jinan Microecological Biomedicine Shandong Laboratory is the co-first author of this paper. Professor Cao Yi and Associate Professor Xue Bin from the laboratory are the co-corresponding authors. The Microecology New Technology Centre, where Academician Gu Xiaosong and Deputy Director Jiang Chunping's team are based, participated in the research related to this achievement. This research was supported by projects from the Jinan Microecological Biomedicine Shandong Laboratory (project numbers: JNL-2025008B, JNL-2025009B, JNL-2025010B) and the Shandong Provincial Laboratory Project (project number: SYS202202).