Recently, addressing the key bottleneck of limited clinical application of traditional STING agonists (such as the need for intratumoral injection and significant systemic side effects), Professor Wang Hangxiang's team was invited to publish a Spotlight review article titled ‘Repurposing of old drugs for unleashing innate immunity’ in the top-tier immunology journal *Trends in Immunology*. The review paper delves into a groundbreaking study that repurposes the old drug carvedilol to allosterically sensitise the STING pathway. The study discovered that carvedilol, a drug that has been on the market for years, can activate anti-tumour immunity through a novel regulatory mechanism involving a new signalling protein pathway, potentially addressing the issue of drug resistance in STING-targeted anti-tumour therapies.

Activating the STING pathway is a highly promising therapeutic strategy for anti-tumour immunity. As a core pathway of the innate immune system, the STING pathway can effectively activate the human immune system to attack cancer cells, thereby transforming ‘cold tumours’ lacking immune cell infiltration into ‘hot tumours’ with abundant immune cell infiltration. However, traditional STING agonists require intratumoral injection for clinical use, limiting their clinical application. Systemic administration faces significant challenges such as systemic immune side effects, drug delivery difficulties, and poor pharmacokinetics. This has led to no STING agonist being approved for market release globally to date, making the development of safe and effective STING drugs of significant clinical value.
This Spotlight paper focuses on a recent study by Dang et al. published in Cell Reports. The study identified, through large-scale high-throughput screening, that carvedilol—a cardiovascular drug that has been safely used in clinical settings for years—can regulate the STING pathway in a novel manner (Figure 1). Unlike existing STING agonists, carvedilol acts as a signal pathway amplifier. Carvedilol does not directly activate the STING pathway but binds to an allosteric site (T263) on the STING protein. Additionally, carvedilol selectively and efficiently amplifies STING activation only when tumour cells release endogenous activating signals (such as cGAMP). This conditional activation mode enables precise targeting of the tumour microenvironment, potentially avoiding the inflammatory cytokine storm and other toxic side effects associated with traditional agonists.
Furthermore, this Spotlight article delves into the key mechanisms underlying the antitumour effects of this novel therapeutic strategy in vivo. The antitumour efficacy does not rely on traditional CD8+ T cells but instead achieves tumour clearance by strongly activating and recruiting the first line of defence of the innate immune system—natural killer cells. This discovery holds significant clinical implications, potentially offering effective treatment options for ‘cold tumours’ that lack T cell infiltration and are unresponsive to existing immune checkpoint inhibitors.
This study provides new insights and strategies for STING-targeted drug development—shifting from broad-spectrum activation to precise regulation. As a marketed drug with established safety, the ‘repurposing’ strategy of carvedilol offers greater feasibility for rapid clinical translation and also provides new approaches for designing safer and more efficient allosteric immunomodulators.
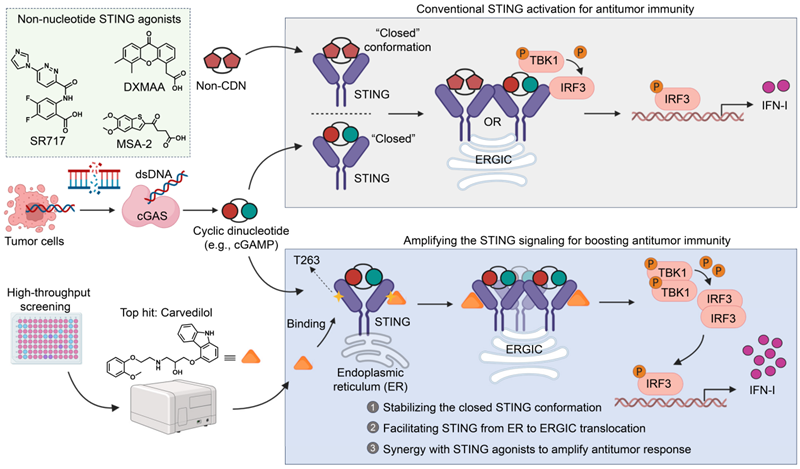
Figure 1: Carvedilol: A novel STING pathway signal-sensitising drug. Dang et al.'s research breaks through the traditional working mode of STING agonists. Conventional STING activation mechanisms (as shown in the upper figure) rely on ligands such as cyclic dinucleotides (e.g., cGAMP) or synthetic cyclic dinucleotide analogues (e.g., DXMAA, MSA-2, or SR717), which bind to the orthosteric site to trigger downstream signal transduction and the production of type I interferons. However, Dang et al.'s study indicates that carvedilol acts as an allosteric modulator (lower figure). This compound binds to the T263 amino acid residue, stabilising the active conformation of STING, promoting its transport from the endoplasmic reticulum to the endoplasmic reticulum-Golgi intermediate compartment, and synergistically amplifying the type I interferon response initiated by the initial signal. Abbreviations: cGAMP, cyclic guanosine monophosphate; cGAS, cyclic guanosine monophosphate synthase; dsDNA, double-stranded DNA; ER, endoplasmic reticulum; ERGIC, endoplasmic reticulum-Golgi intermediate compartment; IFN-I, type I interferon; IRF3, interferon regulatory factor 3; STING, stimulator of interferon genes; TBK1, TANK-binding kinase 1.
Key points: As a safe and well-known marketed drug, carvedilol selectively amplifies STING activation only when endogenous signals are present in the tumour microenvironment through a unique ‘allosteric sensitisation’ mechanism, providing a novel and highly promising approach for the development of safe and effective systemic STING-targeted therapies.
The Wang Hangxiang research group has long been engaged in basic and clinical translational research on anti-tumour immunotherapy drugs and delivery systems. In recent years, they have achieved some成果 in the field of STING agonist-related drug formulation research, publishing multiple papers in journals such as Cancer Research and Nature Communications. The team has published over 100 papers in academic journals such as PNAS, Nat. Commun., Cancer Res., Adv. Mater., ACS Nano, Biomaterials, and J. Controlled Release. This research was supported by the Shandong Provincial Natural Science Foundation (ZR2023ZD59) and the Jinan Microecological Biomedicine Shandong Laboratory Research Project (JNL-2025007B), among others.