Recently, Professor Hangxiang Wang's team has proposed a new drug delivery strategy based on vitamin C, which is safe, reliable and can be taken for a long period of time, in order to address the difficulty of treating inflammatory bowel disease (IBD) effectively due to the rapid metabolism of vitamin C (VC) in the body. By coupling vitamin C with long-chain fatty acids, the team successfully developed a small-molecule self-assembled drug system, which significantly enhanced the enrichment and therapeutic effect of vitamin C at the site of inflammation, and is expected to provide a new solution for the clinical treatment of inflammatory bowel disease (IBD). This study was published in the Journal of Nanobiotechnology under the title ‘Reduction of colitis in mice by chemically programmed supramolecular nanoassemblies of vitamin-lipid conjugates’. It was published in the Journal of Nanobiotechnology (IF: 10.6, CAS Region I) under the title ‘Reduction of colitis in mice by chemically programmed supramolecular nanoassemblies of vitamin-lipid conjugates’.

Inflammatory bowel disease (IBD), including ulcerative colitis and Crohn's disease, is a complex disease triggered by chronic inflammation in the gastrointestinal tract, and about more than 10 million people suffer from IBD worldwide. With the improvement of living standards and changes in dietary environment, the incidence of IBD in China has also increased significantly, and is expected to reach 1.5 million by 2025, and the incidence of the population is on the younger side. Prolonged inflammatory response can lead to damage to the intestinal barrier, increase the contact between intestinal microorganisms and the immune system, further aggravate the inflammatory response, and may even induce serious complications such as colorectal cancer. Currently, the main treatments for IBD include immunosuppressants, biologics and antibiotics. However, the efficacy of these treatments is limited and their long-term use can trigger side effects such as infection and malignancy. Therefore, a more effective and safer treatment strategy is urgently needed.
Vitamin C is a natural antioxidant that reduces oxidative stress and improves inflammatory response by scavenging reactive oxygen species (ROS). However, the rapid metabolism of vitamin C as a dietary supplement in vivo and other characteristics limit its application as a therapeutically active molecule, specifically: poor stability and easy degradation; rapid metabolism in vivo with a short half-life; and difficulty in effective enrichment at sites of inflammation. In order to solve the above application problems of vitamin C, the team developed a ‘drug coupling and self-assembly’ technology platform, through the covalent coupling of vitamin C with lipid long-chain fatty acids, so that the lipid coupling molecules can be self-assembled to form nanoparticles in aqueous solution, in order to enhance the metabolism resistance of vitamin C, and to improve the bioavailability and targeted accumulation capacity. accumulation ability.
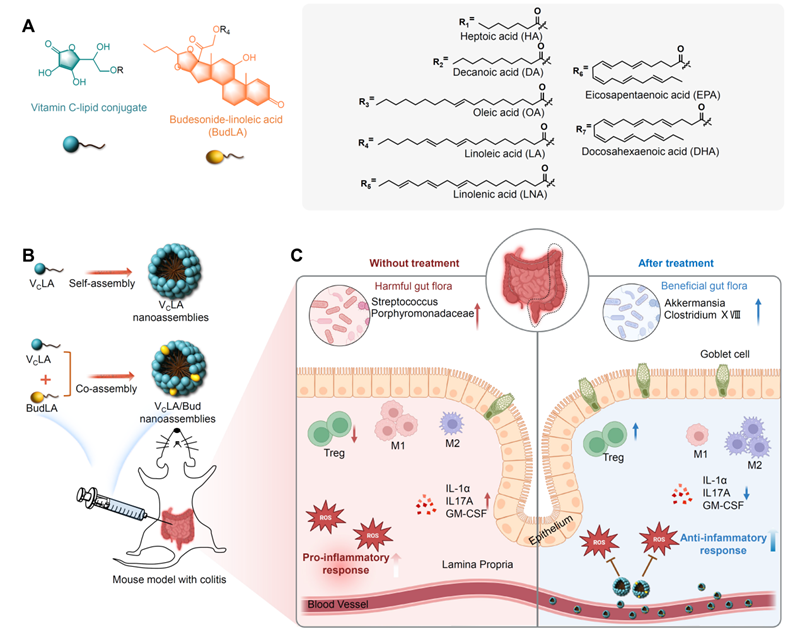
Fig. 1. Design, self-assembly and use of lipid amphiphilic molecules of vitamin C for efficient and safe in vivo drug delivery and inflammatory bowel disease therapy.
In this study, vitamin C was coupled with various fatty acids by esterification reaction to form amphiphilic lipid molecules (i.e., having both hydrophilic and lipophilic properties). These molecules were able to spontaneously form nanoparticles in aqueous solution, thereby improving vitamin C's resistance to degradation and enhancing its targeting delivery efficiency at sites of inflammation. The nano-assemblies (VCLA) formed by linoleic acid (LA) and vitamin C were found to have optimal stability and ROS scavenging ability through pre-screening and characterisation. Experimental results in a mouse colitis model showed that VCLA nano-assemblies could be effectively enriched at the site of inflammation, significantly reducing ROS levels, decreasing intestinal damage and alleviating the inflammatory response.
In addition, a combined drug delivery and therapeutic system was developed by integrating the clinically used glucocorticoid Budesonide (Bud) into the VC nano-self-assemblies. Compared to vitamin C or budesonide alone, the combination therapy demonstrated more significant efficacy in reducing inflammation and protecting the intestinal barrier, without causing significant suppression of the systemic immune system.
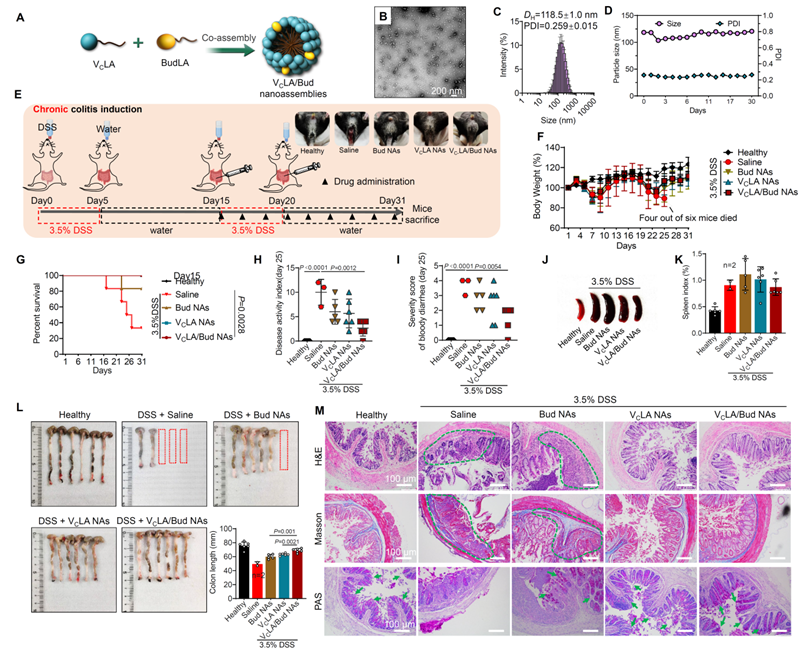
Figure 2. Therapeutic effect and pharmacodynamic evaluation of budesonide/VC nanoself-assemblies in chronic inflammatory bowel disease. By intraperitoneal administration, the combined self-assembled body significantly alleviated intestinal inflammation and protected the intestinal barrier.
Further mechanistic studies showed that the budesonide/VC self-assembler drug effectively remodelled the intestinal inflammatory microenvironment by decreasing the number of pro-inflammatory M1-type macrophages and increasing the proportion of anti-inflammatory M2-type macrophages. Enrichment of the drug at the site of inflammation was significantly enhanced by targeted delivery, reducing systemic side effects. In addition, 16S rRNA sequencing results showed that the intestinal flora of mice with chronic enteritis was significantly different compared with that of healthy mice; and the self-assembled body drug significantly improved the intestinal flora, making it more inclined to that of healthy mice. In particular, some beneficial bacteria such as Akkermansia Akkermansia and Clostridium Clostridium XVIII were significantly increased in the intestinal tract of the drug-treated mice, while a significant down-regulation of Streptococcus spp. Streptococcus was also found.
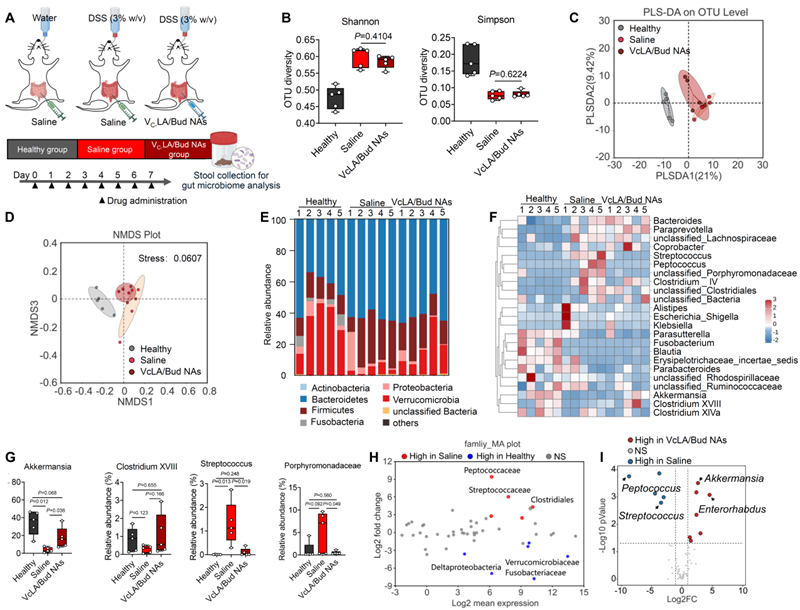
Fig. 3. Budesonide/VC nanoself-assemblies modulating intestinal flora.
In this study, we proposed a small molecule self-assembled drug delivery system with simple synthesis and preparation and high scalability, which provides a new idea for the clinical application of vitamin C. This strategy not only addresses the poor pharmacokinetics of vitamin C in vivo, but also significantly enhances its anti-inflammatory effect by targeting the inflammatory site for enrichment and slow release. In addition, through modular design, it can be combined with other anti-inflammatory drugs to further expand its potential application in other inflammatory diseases.
It is worth mentioning that the vitamin C-coupled molecule is synthesised in a simple step, and only vitamin C and polyunsaturated fatty acids are produced after degradation, so it is safe and is expected to have a good prospect for clinical application and translation.
Scientific Inspiration
This innovative strategy overcomes the limitations of vitamin C as a therapeutically active molecule and demonstrates good anti-inflammatory effects and safety in mouse models, providing new ideas for the treatment of IBD and other inflammatory diseases.
The study was carried out by the Jinan Microecological Biomedicine Shandong Laboratory in collaboration with the First Hospital of Zhejiang University School of Medicine, with the JJinan Microecological Biomedicine Shandong Laboratory as the first completion unit. This work was supported by the project of Jinan Microecological Biomedicine Shandong Laboratory(No. JNL-2022010B and JNL-2025007B), the Major Basic Research Project of Natural Science Foundation of Shandong Province, and the top-level project of National Natural Science Foundation of China.