Recently, the team of Prof. Li Xinguang, Deputy Director of the Laboratory, and Researcher Chen Jingjing published a paper entitled ‘SUMOylation, modification of HNRNPK at the K422 site promotes invasion in glioblastoma’ in the international journal of biological sciences, International Journal of Biological Sciences (CAS Top 1, IF=8.2). modification of HNRNPK at the K422 site promotes invasion in glioblastoma". This study reveals that SUMOylation modification of HNRNPK at the K422 site of glioma RNA/DNA binding proteins (RBPs) interferes with its DNA-binding ability, which disrupts the downstream transcriptional regulation and ultimately results in the transformation of glioblastoma stem cells (GSC) into preneuronal subtypes (PN-types) and promotes tumour invasion.

Gliomas, as adult intracranial malignant tumours, have extremely high rates of disability and mortality, among which Glioblastoma (GBM) is the most malignant glioma.The highly heterogeneous nature of GBM tumours, and the ability of different cell types to adapt to their surroundings and undergo transformations significantly increase the complexity of the study and treatment of GBM, and this is the poor clinical outcome of GBM the main reason for the poor clinical outcome of GBM. The team is committed to identifying and profoundly influencing the key RNA/DNA binding proteins (RBPs) that contribute to GBM genesis and development, providing new strategies for the understanding and treatment of GBM.
Protein SUMOylation (SUMOylation) is a fine-grained post-translational modification that plays an important role in numerous cell biological processes such as cell cycle regulation, cell metabolism, gene transcription, etc., a process that intimately regulates the key processes of tumour progression, metastasis, and treatment resistance. The thesis team has conducted an in-depth study to address the key scientific questions within this field, relying on high-throughput sequencing technology and single-cell analysis, using phosphorylated protein microarrays, surface plasmon resonance (SPR), and other technological tools, and original multicolour fluorescence 3D reconstruction and depth of invasion analysis, combined with an in vivo animal model.
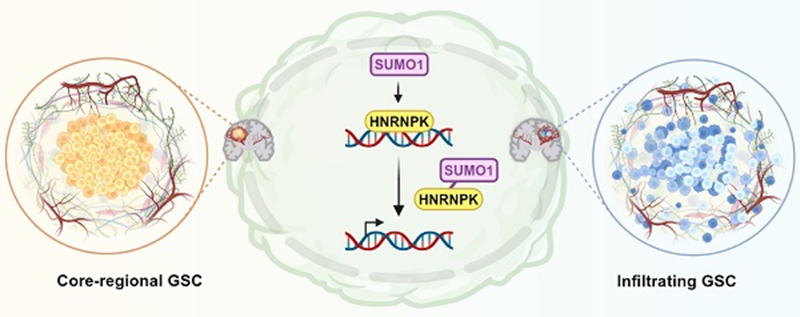
In GBM, we found that the SUMOylated modification of the RBPs protein HNRNPK was found to be highly expressed in the proliferative regions of the tumour microvasculature and tended to be expressed in neural progenitor cell-like (NPC-like) tumour cells.HNRNPK-SUMO1 expression was found predominantly in the infiltrating regions of the GBM.SUMOylated modification of the K422 residue of HNRNPK interfered with its DNA binding ability, thereby disrupting downstream transcription and ultimately leading to transitions between glioblastoma stem cell states. This finding provides a new molecular target for the treatment of GBM, which may inhibit the invasive ability of GBM cells by regulating the SUMOylated modification status of HNRNPK, and is clinically important for the development of new therapeutic tools for GBM.
Scientific Inspiration
The team has published several representative articles in Clinical Cancer Research, Theranostics, etc. around the research field of RBPs in glioma in the previous period. This study highlights the potential of dynamic regulation of RBPs and their SUMO-enabled modifications in the tumour microenvironment, which provides important insights for the development of precision therapeutic strategies against the transformation of tumour cell states, and also provides theoretical support for innovative research on targeted cancer therapies.
This study was supported by grants from the National Natural Science Foundation of China (82472932), Taishan Scholars Project of Shandong Province (tsqn201909173), Major Basic Research of Shandong Province (ZR2022ZD36), and Jinan Microecological Biomedicine Shandong Laboratory Project (JNL2023007C and JNL-2022003A).