Recently, the team of Academician Xiaosong Gu, Deputy Director of Jinan Laboratory, Prof Chunping Jiang and PI Junhua Wu published an important research result in Cell Death and Differentiation with the title ‘SENP3 inhibition suppresses hepatocellular carcinoma progression and improves the efficacy of anti-PD-1 immunotherapy’. SENP3 inhibition suppresses hepatocellular carcinoma progression and improves the efficacy of anti-PD-1 immunotherapy’ was published in Cell Death and Differentiation (IF: 13.7, Region I, CAS). The team reported that targeting SENP3 could inhibit the malignant progression of hepatocellular carcinoma and enhance the efficacy of anti-PD-1 immunotherapy.
Hepatocellular carcinoma (HCC), as the most predominant pathological subtype of primary liver cancer (accounting for more than 90% of all primary liver cancers), has become one of the most important causes of cancer deaths worldwide. The early clinical manifestations of the disease are insidious, and about 70% of the patients are diagnosed with advanced stages of the disease, which require systemic drug therapy. Current clinical practice is based on the combination of immune checkpoint inhibitors and tyrosine kinase inhibitors as the core regimen, but there is significant heterogeneity in individual efficacy, which highlights the need for in-depth analysis of the molecular mechanisms of HCC.
SUMOylation, a highly conserved post-translational modification mechanism, induces reversible binding of small ubiquitin-like modifiers (SUMOs) to substrate proteins through a cascade of SUMO-activating enzyme (SAE), conjugating enzyme UBC9, and specific E3 ligases, which in turn regulates their enzymatic activity, subcellular localisation, protein stability, and interactions network. This modification system is widely involved in key biological processes such as cell cycle regulation and DNA damage repair, and is closely related to the development of various malignant tumours. Notably, SUMO pathway-targeted drugs (e.g., TAK-981, momordin Ic) have shown clinical translational potential in solid tumour therapy, but the molecular mapping of SUMOylation in the shaping of the malignant phenotype of HCC and its dynamic regulatory mechanisms still urgently need to be systematically elucidated.
SENP3 (Sentrin/SUMO-specific protease 3), small ubiquitin-like modifier (SUMO)-specific desumoylase 3, is a protease that plays a key role in SUMOylation. It efficiently releases SUMO2 and SUMO3 from substrate proteins.SENP3 regulates a variety of cellular processes through de-SUMOylation, including gene expression, signalling and stress responses.
The team demonstrated that targeting SENP3 can inhibit the progression of hepatocellular carcinoma and enhance the effect of anti-PD-1 therapy, and delved into its molecular mechanism, suggesting that SENP3 has significant potential as a new therapeutic target to enhance the responsiveness of HCC to immunotherapy, providing a theoretical basis for the development of new therapeutic strategies for HCC.
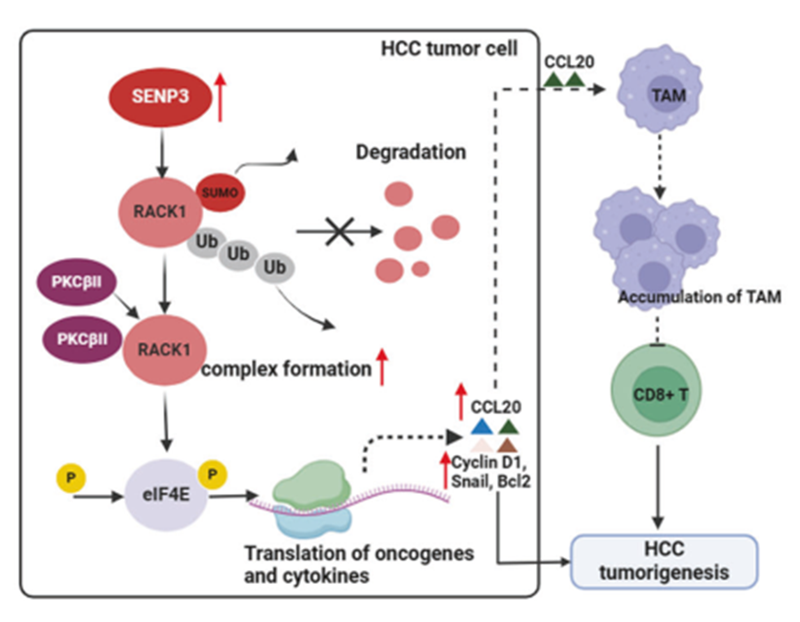
By screening SENP family members, the team found that SENP3 is highly expressed in HCC and associated with poor prognosis. Then, ex vivo and in vivo functional experiments further confirmed that SENP3 promotes the proliferation, invasion and migration of tumour cells, while inhibiting apoptosis.
In addition, this study showed for the first time that SENP3 can specifically desumoform RACK1 and enhance its stability and binding ability to the synergistic molecule PKCβII, leading to an increase in the phosphorylation of eIF4E, and affecting the translation of downstream pro-tumorigenic factors, such as BCL2, Snail, and Cyclin D1.
It has been reported that the malignant phenotypic changes in the tumour cells themselves due to genotypic alterations within the tumour cells may not be consistent with the immune microenvironmental alterations they cause, and the immune microenvironmental alterations induced by the phosphorylation of eIF4E have already been reported, so this study also explored whether the altered expression of SENP3 in hepatocellular carcinoma cells would affect the immune microenvironment. In this study, it was first found that SENP3 promoted the migration of tumour-associated macrophages into the tumour and reduced the number of infiltrating CD8+ T cells; and targeting SENP3 in combination with anti-PD-1 treatment produced a significant tumour-suppressive effect.
Cytokine array showed that SENP3 could affect the expression of CCL20, and further investigation revealed that SENP3 could regulate the translation of cytokine CCL20 through the RACK1/eIF4E axis, thus affecting the migration of tumour-associated macrophages.
Taken together, SENP3 drives hepatocellular carcinoma progression by regulating the malignant phenotype and immunosuppressive microenvironment of hepatocellular carcinoma cells. Mechanistically, SENP3 inhibits SUMOylation of RACK1 at residues K264 and K271. This increases the stability of RACK1 and its ability to synergise with PKCβII to promote translation of BCL2, Snail, Cyclin D1 and CCL20.
Inspiration: This study provides a key scientific basis for the development of novel molecular targeted therapies, and significantly improves the optimisation pathway of immunotherapy response by revealing the new mechanism of immunoregulation in the tumour microenvironment, and its innovative discoveries will powerfully promote the clinical translation and application of precise and individualised therapeutic strategies in hepatocellular carcinoma, and provide important theoretical basis and practical direction for the optimisation and innovation of hepatocellular carcinoma clinical therapeutic strategies.
This study was supported by the Shandong Provincial Laboratory Project (No. SYS202202), the National Natural Science Foundation of China (No. 81972888, 82272819), the Provincial Key Research and Development Programme (No. BE2018701, BE2022840), and Jinan Microecological Biomedicine Shandong Laboratory (No. JNL2022004A, JNL2022019B). JNL2022019B, JNL2022017D).
Original link: https://doi.org/10.1038/s41418-024-01437-9.