On 21 October 2024, the team of PI Prof. Yi Cao and full-time assistant researcher Jiapeng Yang from Jinan Laboratory published a research article entitled ‘Photo-Tunable Hydrogels Reveal Cellular Sensing of Rapid Rigidity Changes through Accumulation of Mechanical Signaling Molecules’. Using hydrogels with photoresponsive rigidity, the team revealed a frequency-dependent response of cells to stiffness changes in a cycling dynamic mechanical environment. Strikingly, at some frequencies, the traction of the cells even exceeds that on a static substrate that is four times stiffer, challenging existing models of molecular clutches. It was found that the discrepancy between the rapid adaptation of traction and the slow inactivation of force-transducing signalling proteins leads to an accumulation of signalling proteins that enhance the long-range traction of cells in dynamic environments. Based on this, the team proposed a new model that incorporates immediate force transmission and extended signalling, revealing that cells sense periodic changes in extracellular matrix stiffness through the accumulation of signalling proteins, which in turn enhances their traction. This study emphasises the critical role of dynamic stiffness in the development of synthetic biomaterials, highlighting the need to consider both immediate and long-term cellular responses.
In tissues, cells are in a dynamic microenvironment consisting of other cells and the extracellular matrix (ECM). They interact mechanically with the microenvironment through adhesion patches (FAs) that not only exert traction forces but also sense mechanical changes in the external microenvironment. Typically, force sensing by cells is studied in static environments, such as on soft materials, where the cells exert weak traction forces.
However, the mechanical properties of the microenvironment can change over time, especially during tissue development or disease. Even daily activities such as heartbeat, respiration, and digestion introduce periodic mechanical fluctuations that are critical for cellular function. Although the molecular clutch model (MCM) effectively explains how cells exert more force on harder substrates, it still has limitations in explaining the adaptation of dynamic mechanical fluctuations in tissues and organs.
Based on the current research limitations, this study developed a light-responsive hydrogel that can rapidly and reversibly switch between hard and soft states. It was shown that rapid periodic changes in substrate hardness (1-min intervals, 28% change in hardness) were able to enhance the traction of the cells even more than on a 4-fold harder but static surface.
Further studies found that this enhancement was associated with the phosphorylation of signalling proteins such as intracellular adhesion patch kinase (FAK), force signalling proteins that can persistently activate and accumulate after rapid changes in extracellular matrix stiffness, enhancing cellular traction. The study also developed a computational model that explains how cells integrate dynamic mechanical signals to regulate their behaviour and fate.
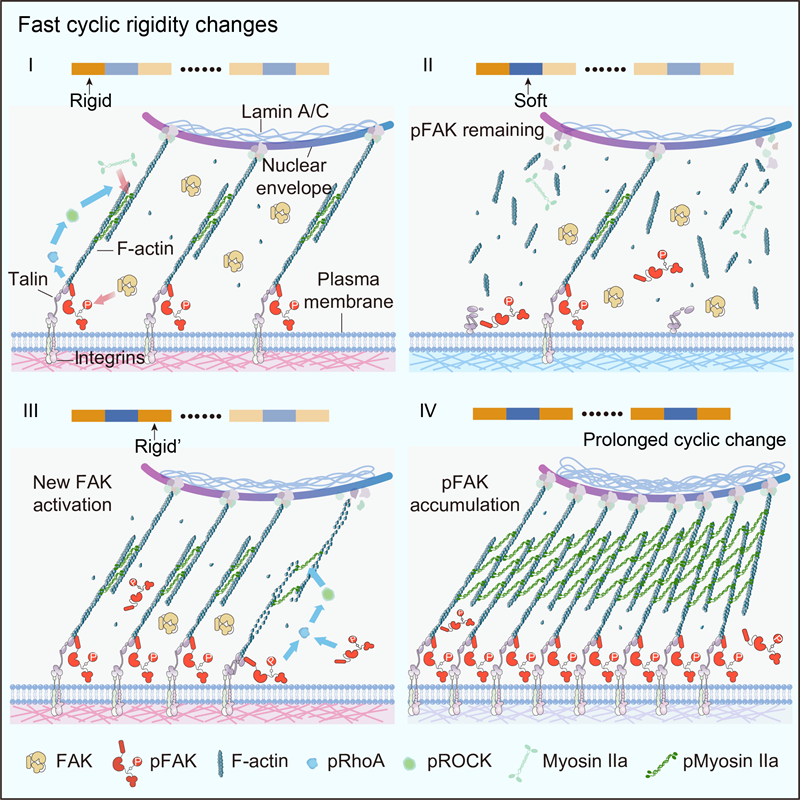
The research team explored the effects of rapid stiffness changes on cellular force sensing and signalling with a novel photoresponsive hydrogel (PYP hydrogel) prepared by the research team. The results showed that rapid matrix stiffness changes significantly enhanced cellular traction and facilitated force signalling. Further analyses showed that when the matrix was softened, molecular clutches dissociated, leading to the detachment of signalling molecules (e.g. pFAK) from molecular clutches or integrin clusters and their entry into the cytoplasm.
Notably, the detached force signalling molecules are not immediately dephosphorylated and still maintain force signalling effects. When the matrix rapidly regains stiffness, these phosphorylated state signalling molecules reattach to the reassembled molecular clutches, leading to an accumulation of force signalling molecules, which further enhances cellular traction. pFAK's slow rate of dephosphorylation during the softening phase and its rapid rate of phosphorylation during the stiffness restoration phase are key factors in the enhancement of force signalling transduction.
This study demonstrates that cellular force signalling is a complex process that spans multiple time scales, with force transmission typically occurring in seconds to minutes and the phosphorylation and dephosphorylation of signalling molecules occurring more slowly.
Scientific Inspiration
In future studies, the team will delve deeper into these time-scale interactions to further understand how cells translate dynamic force signalling into specific biological outcomes. This finding has important implications for active biomaterial design and tissue engineering applications.
Jinan Microecological Biomedicine Shandong Laboratory was the first to complete the paper, with Jiapeng Yang, assistant researcher of JPLMB, as the first author, and Prof Yi Cao, PI of JPLMB, and other co-corresponding authors. This study was supported by the National Key Research and Development Programme of China (No. 2020YFA0908100), the National Natural Science Foundation of China (No. T2222020, T2225016 and 11934008), the Shandong Provincial Laboratory Project (No. SYS202202), and the research projects of the Jinan Microecological Biomedicine Shandong Laboratory (No. JNL2022019B and JNL2023004Q) and the Natural Science Foundation of Jiangsu Province (No. BK20220120).
Original link: https://doi.org/10.1016/j.stem.2024.09.016