On 1 October 2024, the team of Academician Xiaosong Gu, Deputy Director of Jinan Laboratory, Professor Chunping Jiang and PI Junhua Wu published an important research result in Cell Reports Medicine with the title ‘Thymosin a1 reverses oncolytic adenovirus-induced M2 polarization of macrophages to improve antitumor immunity and therapeutic efficacy’. Thymosin a1 reverses oncolytic adenovirus-induced M2 polarisation of macrophages to improve antitumor immunity and therapeutic efficacy’ was published in Cell Reports Medicine (IF: 11.69, Region I, Chinese Academy of Sciences). The team reported that thymosin a1 reverses the negative immune feedback induced by oncolytic adenovirus - macrophage M2 polarisation and regulatory T-cells (Tregs) expansion - and thus improves anti-tumor immunity and therapeutic efficacy.

Oncolytic viruses (Ovs) are a novel class of immunotherapeutic agents that can promote tumour regression by preferentially replicating in tumour cells, inducing immunogenic cell death and stimulating host anti-tumour immunity. Adenoviruses are one of the most widely studied and promising OVs, and a large number of clinical trials with adenoviruses are currently underway.
However, lysogenic adenoviruses typically produce limited antitumour efficacy due to a number of potential limitations of adenoviruses themselves, one of which is the induction of immunosuppressive feedback loops. To date, adenoviral induction of immunosuppressive feedback loops and therapeutic strategies to compensate for these deficiencies remain understudied. Genome-modified replicative type V adenovirus (ADV) therapeutically induces macrophage polarisation to an ‘M2-like’ pro-tumourigenic phenotype and leads to the expansion of regulatory T cells (Tregs).
Thymosin α 1 (Tα1) is a thymus-derived immunomodulatory peptide widely known to modulate the immunoreactivity of innate immune cells such as polymorphonuclear leukocytes, dendritic cells (DCs) and macrophages, and is used as a therapeutic immunomodulatory agent as an adjunct to the treatment of infections, malignant diseases, and the polarisation of ‘buried’ macrophages. Therapy. A recent study demonstrated that Tα1 enhances the efficacy of chemotherapy by reversing cytotaxis-induced macrophage M2 polarisation through activation of the TLR7/SHIP1 axis.
Building on previous findings of conversion of tumour-associated macrophages (TAM) to an M2 phenotype, increased immune infiltration of Tregs in the tumour microenvironment (TME) after ADV treatment, and the ability of Tα1 to regulate immune cells, the team demonstrated that Tα1 can enhance anti-tumour efficacy by reversing ADV-induced immune-suppressor cell expansion. In addition, they designed a recombinant adenovirus lysovirus (ADVTα1) expressing Tα1, which has superior immunomodulatory and anti-tumour efficacy compared with the combination of replicative adenovirus type V (ADV) and thymosin α1 (Tα1) for tumour treatment, and has a good potential for clinical application.
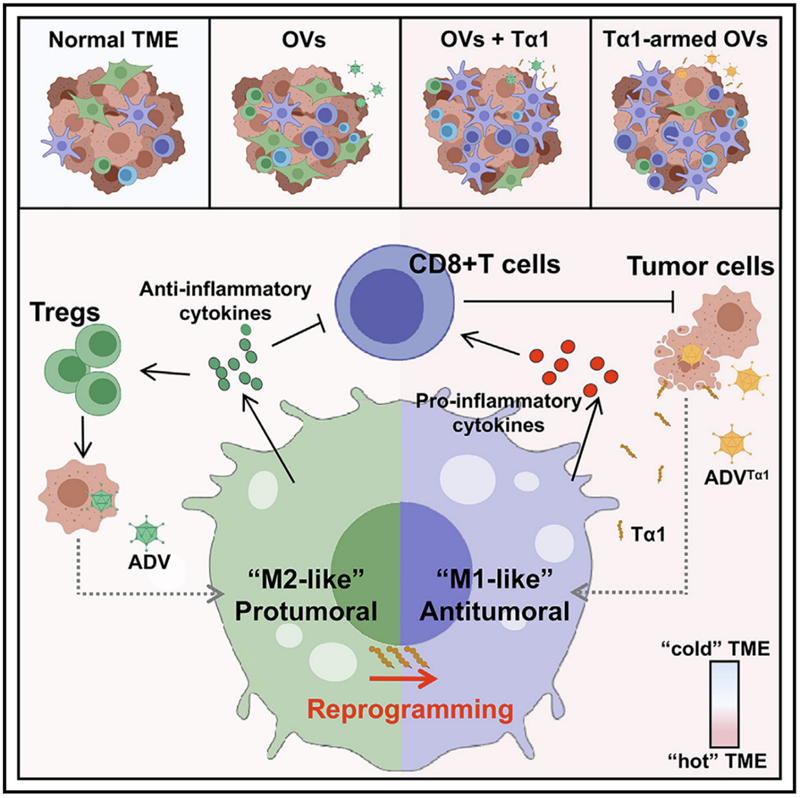
The main players in immunosuppression are Tregs, ‘M2-like’ TAM and innate tumour-associated suppressor myeloid cells. The feedback loop of immunosuppression induced by ADV reduces anti-tumour immunity. More specifically, ADV-infected tumour cells stimulate macrophage polarisation towards ‘M2-like’ pro-tumour phenotypes, leading to the expansion of Tregs within the TME.
TAM in the tumour microenvironment (TME) can be broadly divided into two groups, namely classically activated ‘M1-like’ anti-tumour macrophages and selectively activated ‘M2-like’ pro-tumour macrophages. Classical ‘M1-like’ polarisation is defined by the expression of CD80, CD86, MHCII and iNOS, which are associated with the tumour-killing function of TAM. ‘M1-like’ tumour-associated macrophages phagocytose cancer cells and produce pro-inflammatory cytokines to activate CD8+ T cells for coordinated anti-tumour immunity. In contrast, ‘M2-like’ tumour-associated macrophages are characterised by high expression of CD206, VEGF, CD163, Arg-1 and IL-10, and recruit Tregs to suppress anti-tumour immunity.
In addition, IL-10+TAM has been found to be associated with immunosuppressive TME, where IL-10+ TAM causes cytotoxic CD8+ T cell dysfunction and promotes immune evasion. This study found that in vitro, direct contact between ADV-infected tumour cells and macrophages resulted in high IL-10 expression in macrophages.
In this study, Tα1 reprogrammed ‘M2-like’ tumour-promoting macrophages into ‘M1-like’ anti-tumour macrophages and reduced Treg infiltration in TME during ADV treatment. In addition, the combination therapy showed superior anti-tumour effects compared to a wide range of solid tumours.
Based on the superior antitumour efficacy of the combination of ADV and Tα1, recombinant lysosomal adenoviruses expressing Tα1 have been constructed to efficiently reprogramme TME to a state more conducive to antitumour immunity.Tα1 has been approved in more than 35 countries for the treatment of chronic hepatitis B and C. Tα1 has been shown to be effective in reducing TME infiltration during ADV and Tα1 treatment. Tα1 is not recommended for oncolytic adenoviral therapy because it can induce an antiviral immune response. surprisingly, Tα1 was not found to affect the oncolytic and replicative capacity of ADVTa1 in this study.
In conclusion, ADV-infected tumour cells induce macrophages to adopt an ‘M2-like’ tumour-promoting phenotype in a cell-contact-dependent manner, and the intervention of Tα1 can effectively coordinate macrophage reprogramming, thereby enhancing T cell-mediated anti-tumour responses.
Scientific Inspiration
The team's results showed that the combination of ADV and Tα1 demonstrated superior anti-tumour effects in a variety of solid tumours compared to single-agent treatment, and led to complete tumour elimination and long-term immune memory in H22-loaded mice. Recombinant adenoviruses expressing Ta1 constructed on the basis of the findings effectively reprogrammed the TME to a state more favourable for anti-tumour immunity, with anti-tumour efficacy no less than that of the combination therapy, while having a lighter administration burden. This study provides inspiration for lysosomal virus combination therapy strategies and the design of lysosomal engineered viruses.
This study was supported by grants from the Shandong Provincial Laboratory Project (No. SYS202202), the National Natural Science Foundation of China (No. 81972888, 82272819), and the Scientific Research Project of Jinan Provincial Laboratory of Microecology and Biomedicine (No. JNL2022004A, JNL2022019B, JNL2022017D).
Original link: https://doi.org/10.1016/j.xcrm.2024.101751