Recently, Professor Hangxiang Wang's team from the Microecology and Disease Research Centre of Jinan Microecological Biomedicine Shandong Laboratory has made progress in the field of immune adjuvant research. Aiming at the dilemma of clinical application of STING agonists, the team innovatively proposed the concept of ionizable prodrugs to improve the rapid intracellular activation of immune agonist small molecules and the efficiency of tumour infiltration. This work not only provides new ideas for the design of drug candidates targeting the STING signalling pathway and the development of high-end formulations, but also provides a theoretical basis for the clinical translation of STING agonist injectable formulations. The research result is entitled ‘Ionizable STING-Activating Nanoadjuvants Enhance Tumor Immunogenicity and Potentiate Immunotherapy Efficacy in Solid Tumor’. ‘The paper was published in Cancer Research (IF:12.5).
Immunotherapy, which kills cancer cells by activating the autoimmune system, is one of the main tools in cancer treatment. Immune checkpoint blockade therapy based on CTLA-4 and PD-1/PD-L1 has achieved remarkable results in the clinical treatment of a wide range of tumours. However, the therapeutic efficacy of this approach in ‘immune-desert’ tumours lacking cytotoxic T-lymphocytes remains limited. Therefore, activation of innate immunity in addition to direct targeting of the adaptive immune response could benefit more cancer patients.STING proteins enhance anti-tumour immunity by promoting the maturation of antigen-presenting cells and recruiting CD8+ T cells, and are therefore a highly promising immunotherapeutic target. However, cyclic dinucleotide STING agonists (e.g., cGAMP) present clinical application challenges due to their short half-life, susceptibility to enzymatic degradation or rapid metabolic clearance, and difficulty in cell entry. The new generation of non-nucleotide STING agonists are considered to have better drug-forming properties, but suffer from poor water solubility, and administration by injection usually leads to systemic toxicity and larger adverse reactions.
The group chose the non-nucleotide STING agonist MSA-2 as the research object. Firstly, a series of ionizable prodrugs were designed and synthesized by linking the MSA-2 molecule to a piperazine moiety through carbon chains of different lengths (Figure 1). In the slightly acidic tumour environment, the piperazine moiety in the prodrug molecule was protonated, and this polarity shift promoted the drug to undergo lysosomal escape and penetrate into the cytoplasm, resulting in rapid and effective activation of the STING pathway. By analysing the ability of MSA-2 series of prodrugs to promote BMDC maturation, it was determined that Prodrug 3 (Prodrug 3) had the greatest activity to activate the STING pathway, and therefore Prodrug 3 was used as a model drug for subsequent studies to explore its in vivo delivery and activity. Further, they developed an acid-sensitive liposome (iProsome-3) for intravenous administration of Prodrug 3. In the tumour environment, the liposome was able to promote shedding of the polyethylene glycol shell on its own surface, which shifted the liposome charge from positive to negative, thereby facilitating cellular uptake and generating greater extravascular permeation and tumour tissue penetration than conventional liposomes.
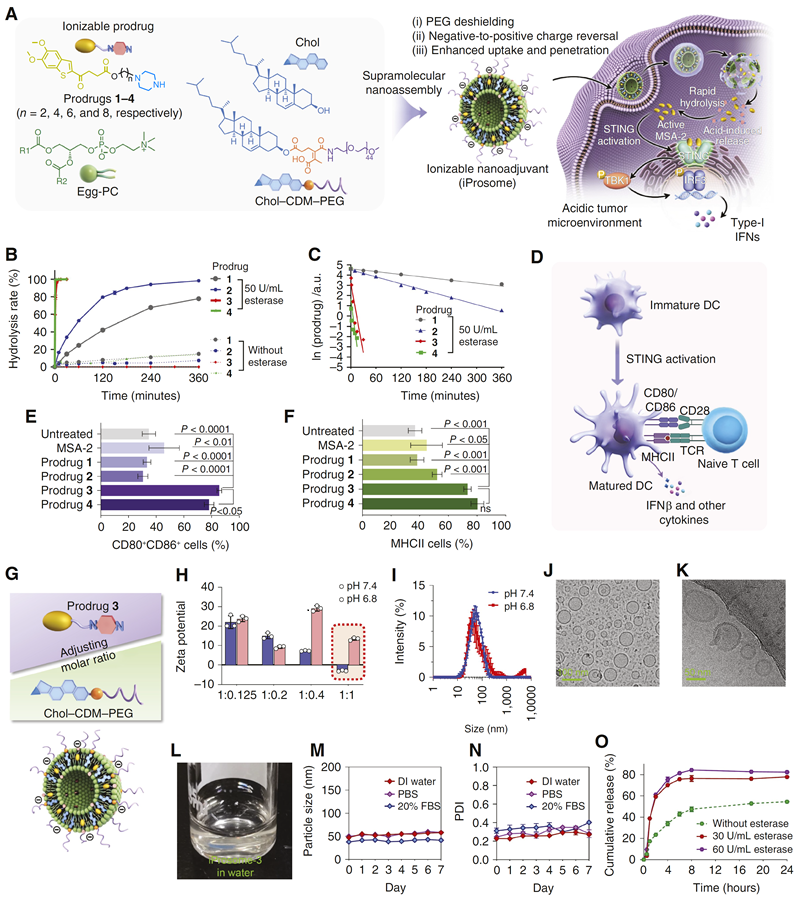
Figure 1, Pre-drug design and construction of nano-adjuvant for MSA-2, an ionisable STING agonist.
In the MC38 colon cancer model, the anti-tumour efficacy of intravenously administered iProsome-3 was significantly higher than that of orally administered free MSA-2, as evidenced by an increase in mature DCs and tumour-infiltrating CD8+ effector T-cells in lymph nodes (Figure 2). In a clinically relevant mouse model of primary colorectal cancer, iProsome-3 showed stronger therapeutic effects than free MSA-2 or conventional liposomes (STING-cLP). In addition, in αPD-1-hyporesponsive 4T1 triple-negative breast cancer, iProsome-3 in combination with immune checkpoint blockade demonstrated excellent tumour suppression. In conclusion, in this study, the group proposed for the first time the design concept of ionizable prodrugs and loaded them onto tumour microenvironment-responsive liposomes to construct nano-adjuvants to efficiently activate the STING pathway-mediated innate immune pathway.
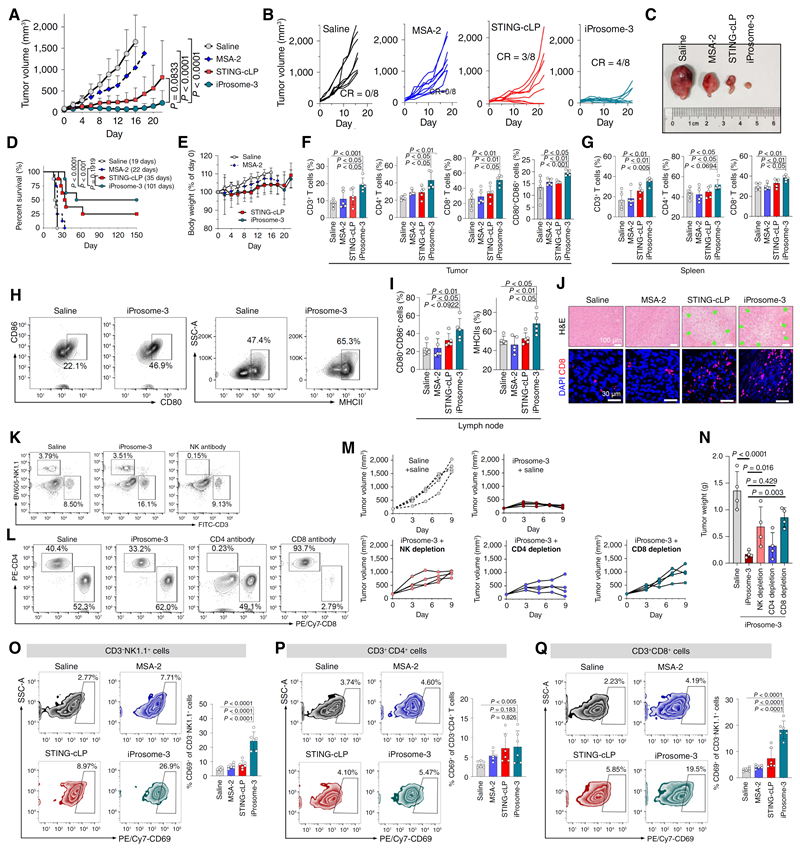
Figure 2, Validation of anti-tumour effect and immune agonistic effect of nano-adjuvant iProsome-3 in MC38 colon cancer model.
Prof Hangxiang Wang's group has published more than 100 papers in academic journals such as PNAS, Nat. Commun., Cancer Res., Adv. Mater., ACS Nano, Biomaterials, J. Controlled Release, etc., and some of the results of the innovative drugs have been transferred. This study was supported by Jinan Microecological Biomedicine Shandong Laboratory (No. JNL-2022010B), the Major Basic Research Project of Natural Science Foundation of Shandong Province and the Top-level Project of National Natural Science Foundation of China.