Recently, Academician Zheng Shusen's team and Professor Wang Hangxiang's team have made the latest progress in the research of drug-free self-assemblies for anti-tumour immune activation, and the research results are entitled ‘Mitochondrial Rewiring with Small-Molecule Drug-Free Nanoassemblies Unleashes Anticancer Immunity’ was published online on 3 September 2024 in the journal Nature Communications (IF: 14.3), with the title “Mitochondrial Rewiring with Small-Molecule Drug-Free Nanoassemblies”. Based on the structural derivation of essential fatty acids, this study developed a drug-free small molecule self-assembler (mtDSN) that induces immunogenic cell death (ICD) and enhances anti-tumour immune response by specifically targeting and modulating mitochondrial function.
Immune checkpoint blockade (ICB) therapy is a revolutionary advancement in the field of anti-tumour therapy and has demonstrated superior clinical efficacy in a wide range of malignancies. However, the cold tumour effect of insufficient tumour T-lymphocyte infiltration, high immunosuppressive factor content and low tumour mutation has resulted in a low response rate to this regimen. Only a small percentage of patients have a clinical immune response to ICB therapy, and many partial immune responders eventually progress due to tumour immune evasion. Stressed and dying tumour cells release a large number of immunostimulatory molecules, including tumour metabolites, tumour-associated antigens and damage-associated pattern molecules (DAMPs). These bioactive components interact with the host immune system and activate adaptive immune responses to induce anti-tumour immune responses.
Studies have shown that mitochondria play an important role in inflammatory regulation and enhancement of tumour immunogenicity, and are potential targets for synergy with current ICB immunotherapies. For example, mitochondrial DNA released after mitochondrial disruption activates the interferon gene-stimulating factor (STING) pathway, in addition to acting as a damage-associated molecular pattern (DAMP), activating the NLRP3 inflammatory vesicle and the Toll-like receptor TLR9 to induce an inflammatory response. In addition, mitochondrial biogenesis, kinetics and reactive oxygen species (ROS) production modulate the function of tumour-infiltrating immune cells.
Therefore, selective mitochondrial targeting and intervention strategies could lead to efficient anti-tumour therapy by stimulating tumour cell immunogenicity, activating specific effector T cells and enhancing their infiltration in the tumour. However, developing therapeutic strategies that promote tumour immunogenicity and increase response to ICB therapy while minimising systemic inflammation and toxicity remains a central challenge for effective immunotherapy.
In this study, by structural derivation based on a variety of saturated and unsaturated fatty acids, the research team developed small molecule constructs of nanoscale self-assemblies (mtDSN). In cellular experiments, we unexpectedly found that this small molecule assemblies could specifically target mitochondria and efficiently induce tumour cell death (Figure 1).
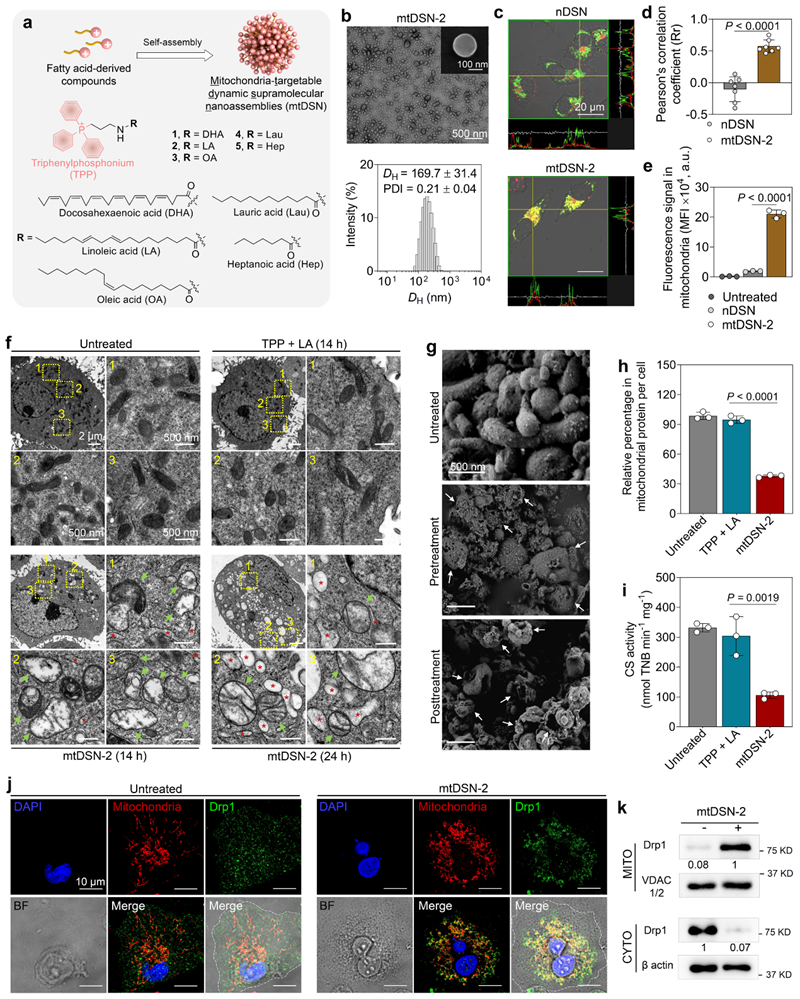
Figure 1. mitochondria-targeted drug-free small molecule nano-self-assembler (mtDSN) induces mitochondrial dysfunction.
Further, the team found that the cell-killing mechanism of this new class of previously unreported ‘non-drug’ small-molecule self-assemblers is significantly different from that of conventional chemotherapeutic drugs. It initiates endoplasmic reticulum stress through aggregation-induced targeted damage to the mitochondrial membrane and leads to apoptosis-like/apoptosis-associated immunogenic cell death (Figure 2).
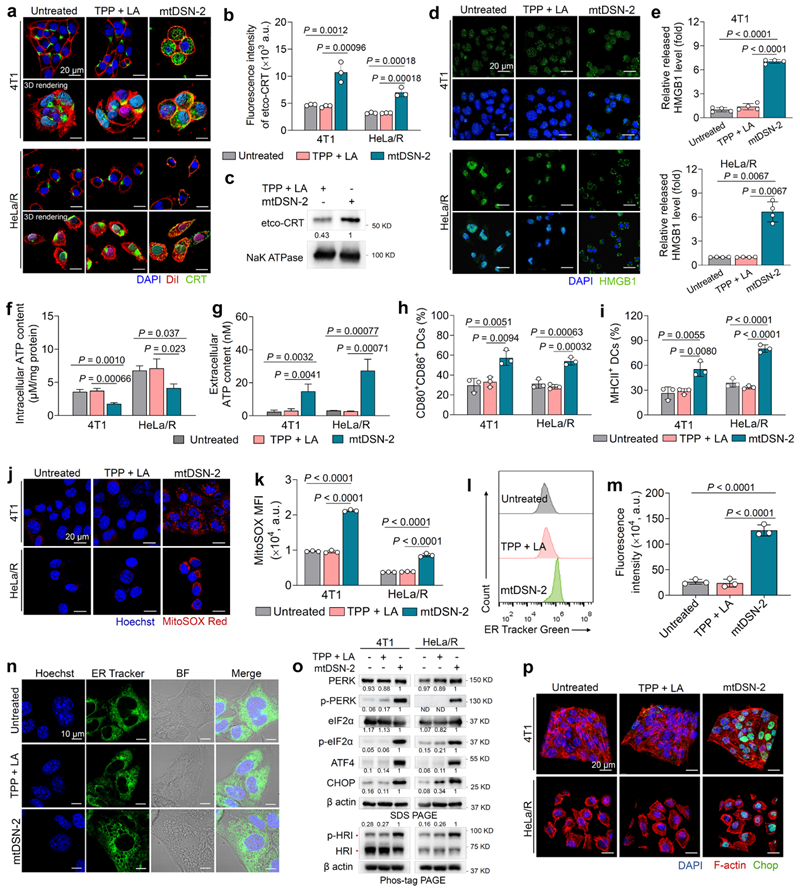
Figure 2. mtDSN-2 induces a stress-related ICD cascade in tumour cells thereby promoting dendritic cell maturation.
After mtDSN treatment, stressed and dying tumour cells can be used as prophylactic or therapeutic tumour vaccines. In a murine model of Holland's tumour with intrinsic or acquired resistance to PD-1 blockade, local mtDSN administration improves the immunosuppressive microenvironment of the tumour, resulting in a shift from a ‘cold tumour’ (low immunogenicity, low T-cell infiltration) to a ‘hot tumour’ (high immunogenicity, high T-cell infiltration, low T-cell activation, etc.). (low immunogenicity, low T-cell infiltration) to ‘hot tumour’ (high immunogenicity, high T-cell infiltration), and synergistically with ICB therapy to produce a highly efficient anti-tumour immune response (Figure 3). The mechanism of action of this chemically optimized small molecule nano-immunity enhancer is different from that of the existing cytotoxic chemotherapeutic agents, providing a new research idea and strategy to regulate the tumour immune microenvironment.
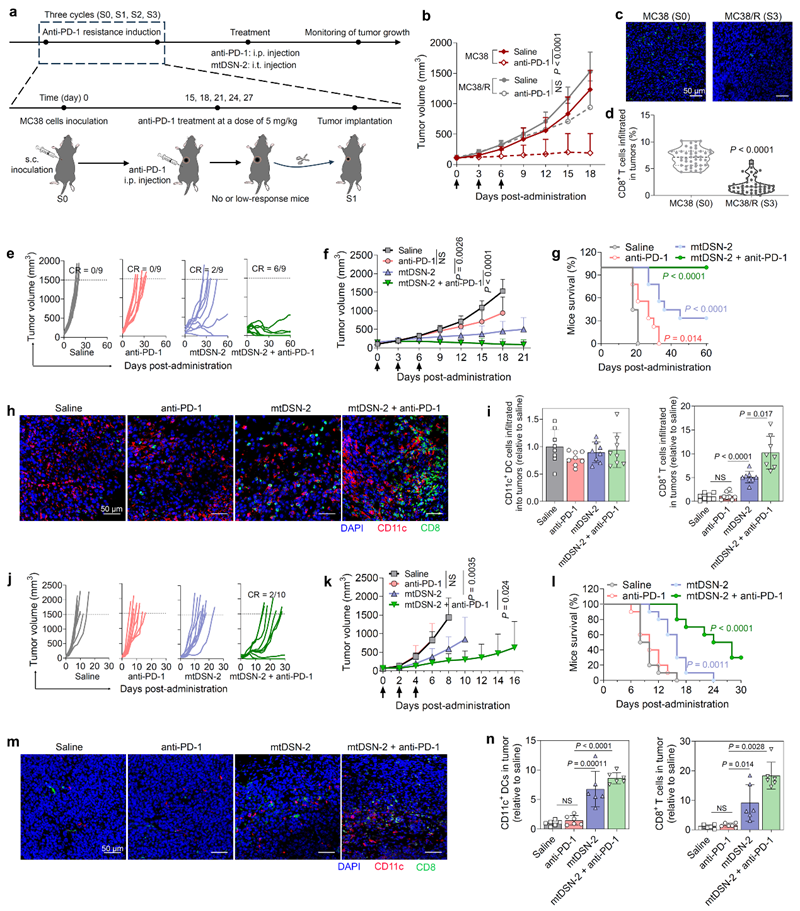
Figure 3. mtDSN-2 activates the tumour immune microenvironment and enhances the anti-tumour immune response of ICB in PD-1-resistant tumours.
Professor Hangxiang Wang's group has long been conducting in-depth research in the field of design and translational application of anti-tumour immune drugs and nano-delivery systems, and has published more than 100 papers in academic journals, such as PNAS, Nat. Commun., Cancer Res., Adv. Mater., ACS Nano, Biomaterials, J. Controlled Release, etc., and some of the innovative drugs are published in the journals of PD-1-resistant tumours. More than 100 papers have been published, and some of the innovative drug results have been transferred and entered the clinical translation stage. This study was supported by the Jinan Microecological Biomedicine Shandong Laboratory (No. JNL-2022010B), the Major Basic Research Project of Natural Science Foundation of Shandong Province, and the Top-level Project of National Natural Science Foundation of China.