In April 2024, the team of Professor Li Xinguang and Researcher Chen Anjing from Jinan Microecological Biomedicine Shandong Laboratory, published their research results in Cancer Letters entitled ‘Targeting the molecular chaperone CCT2 inhibits GBM progression by Targeting the molecular chaperone CCT2 inhibits GBM progression by influencing KRAS stability’. This study addresses the challenges of high malignancy, poor prognosis, and unsatisfactory treatment of glioblastoma (GBM), and aims to find and target the key proteins leading to the occurrence and progression of GBM as a potential strategy for the treatment of GBM.
Protein folding requires the assistance of molecular chaperones, and the study of molecular chaperones of key oncogenes is of great scientific and clinical significance for understanding the mechanisms of cancer occurrence and evolution, and searching for new anticancer therapeutic targets.
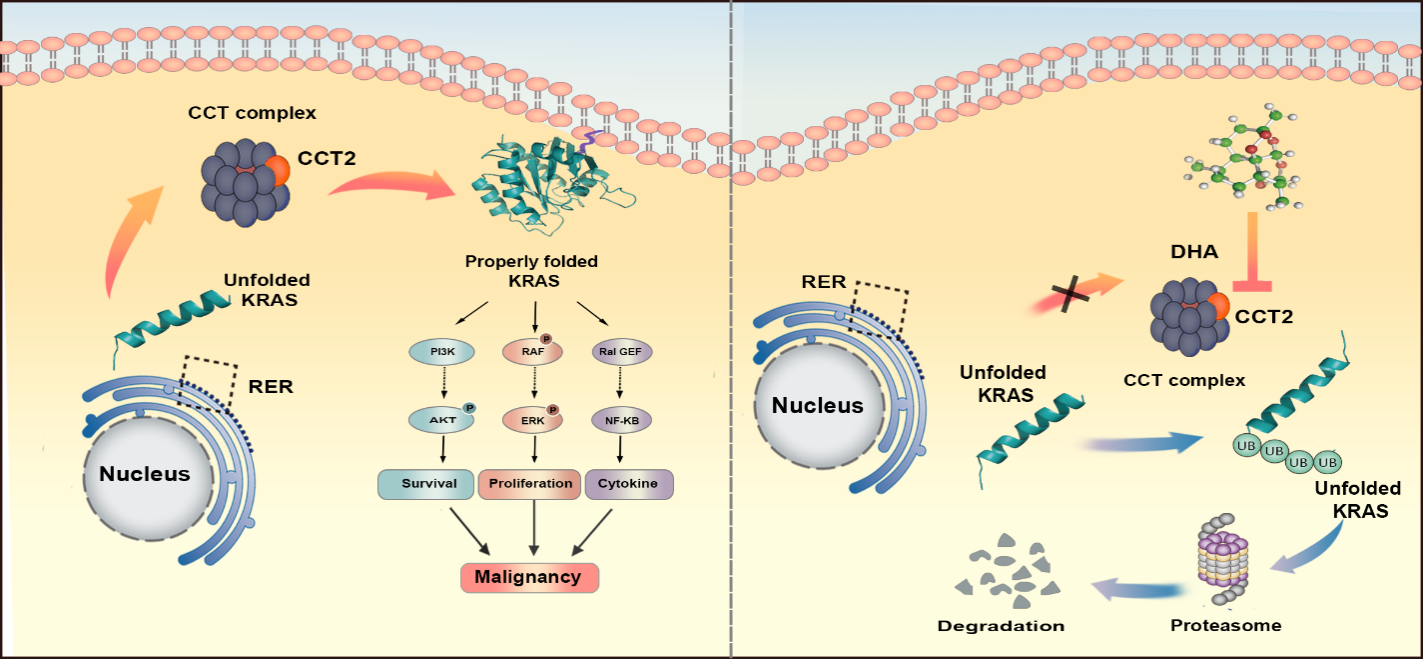
In this study, a highly expressed molecular chaperone, CCT2 (chaperone protein subunit 2 containing the TCP1 complex), was identified in GBM, and the high expression of CCT2 was associated with poor prognosis of GBM as a potential oncogene.
In this study, we demonstrated that CCT2 can promote malignant proliferation and invasive growth of GBM in vitro and in vivo through cellular experiments and by using an in situ xenograft model in nude mice. We also found the downstream molecules that can directly bind to CCT2 by using techniques such as immunoprecipitation (IP), liquid chromatography-mass spectrometry (LC-MS) and surface plasmon resonance (SPR) - - KRAS (Kirsten rat sarcoma virus oncogene homologue), which clarifies the mechanism of CCT2 cancer promotion.
KRAS is a member of the RAS gene, which is mutated in a variety of tumours, leading to continuous activation of downstream signals, which is an important factor in tumourigenesis. Due to its small molecular weight and smooth conformation, KRAS has been synonymous with the term ‘non-druggable’ because it is difficult to target KRAS with small molecule inhibitors.
The team's study demonstrated that CCT2 enhances KRAS downstream signalling and promotes the malignant progression of GBM. Its pro-cancer effect is achieved by binding directly to KRAS and regulating the stability of KRAS.
The team also discovered that dihydroartemisinin (DHA), a first-line drug for malaria treatment, can play a therapeutic role in the treatment of GBM, and that DHA can play an anti-GBM role by directly binding to and inhibiting CCT2, a molecule upstream of KRAS folding and stabilisation, and by targeting CCT2, DHA can avoid the scientific challenge that KRAS is not a drug. DHA is expected to become a targeted drug for the treatment of glioma.
Zhao Feihu, a postgraduate student from Qilu Hospital of Shandong University, is the first author, while Chen Anjing, a researcher from Cell and Neurological Function Testing Platform ofJinan Microecological Biomedicine Shandong Laboratory, and Prof Li Xinguang are the co-corresponding authors. This project was supported by the Taishan Scholars Special Fund Project (tsqn201909173, ts20110814 and tshw201502056), Major Basic Research of Shandong Provincial Department of Science and Technology (ZR2022ZD36), Provincial Laboratory Project of Shandong Provincial Department of Science and Technology (SYS202202), and Jinan Microecological Biomedicine Shandong Laboratory (JNL- 2023007C, JNL-2022003A) and other funds support.