Recently, a team of researcher Hangxiang Wang and Professor Ocean Xie from the First Affiliated Hospital of Zhejiang University School of Medicine were invited by theJinan Microecological Biomedicine Shandong Laboratory to publish a review article titled ‘Nanoparticle-assisted targeting delivery technologies for preventing organ rejection’ in the journal ‘Transplantation’, which is an authority in the field of transplantation. technologies for preventing organ rejection’. The review provides an in-depth description and discussion of the cutting-edge applications of organ- and cell-targeted delivery technologies in organ transplantation, which provide innovative solutions for improving postoperative outcomes and prognosis, and new research ideas for the clinical translation and application of the above delivery solutions.
Organ transplantation allows patients with end-stage organ disease to regain a new life, however, transplant organ damage repair, ischemia-reperfusion injury, anti-rejection, perioperative functional diagnosis and monitoring of transplantation have a direct impact on the effect of organ transplantation, which is closely related to the survival of patients.
The clinical application of immunosuppressive agents (ISA) has greatly improved the success rate of organ transplantation. In recent years, nanobiotechnology has gradually highlighted its unique advantages in the field of transplantation research, especially in the precise drug delivery, targeted (organ and cellular level) immunosuppressive agent delivery research and other aspects of the application of a better prospect for safer and more effective postoperative anti-rejection, which is expected to further improve the success rate of organ transplants and the quality of survival of patients.
The mechanisms of post-transplant rejection can be divided into T cell-mediated rejection (TCMR), antibody-mediated rejection (AMR), and innate immunity-mediated rejection. rejection). The use of immunosuppressive agents is currently the most important and effective means of anti-rejection therapy after transplantation and has been widely used in clinical practice. Therefore, this review firstly summarises the therapeutic targets and anti-rejection mechanisms of immunosuppressive agents currently used in clinical practice (Figure 1).
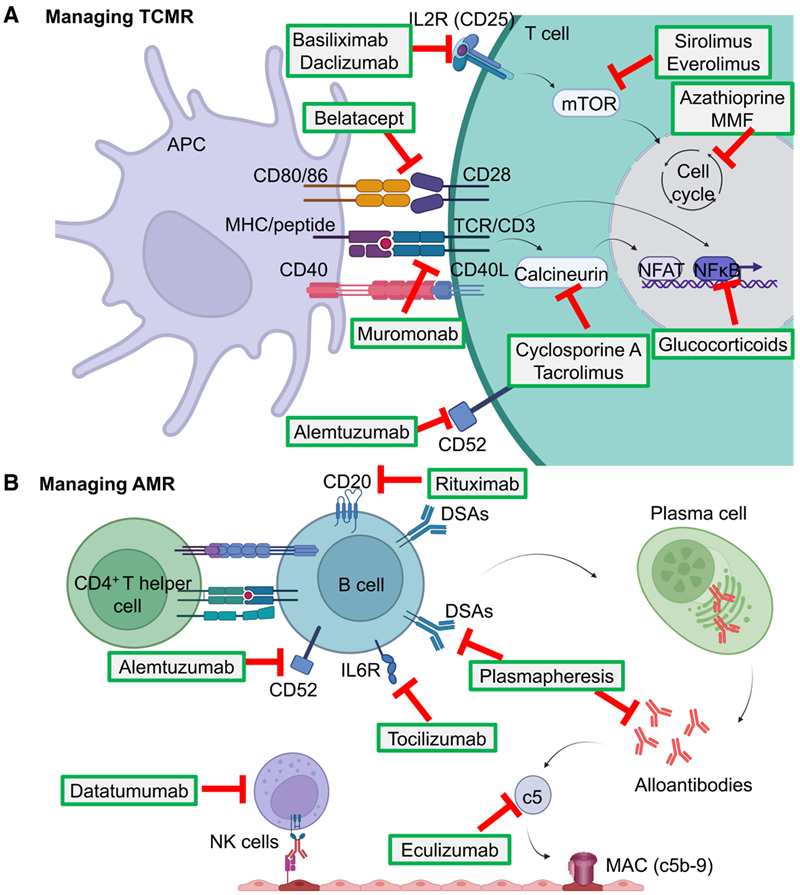
Figure 1, Molecular mechanisms of organ transplant rejection and immunosuppressive agents (ISA).
Although the clinical application of traditional immunosuppressants has effectively improved the survival of organ transplant patients, they still suffer from the drawbacks of high systemic toxicity, risk of tumour recurrence and elevated risk of viral infection.
Novel targeted delivery formulations based on nanotechnology are expected to provide effective solutions to these clinical problems:
(1) Improvement of drug water solubility and pharmacokinetic properties;
(2) Improvement of tissue distribution, specific drug accumulation in target sites (transplanted organs, immune organs or immune cells) and bioavailability;
(3) Lower systemic toxicity. This review summarises the current classification of drug delivery systems and the means of achieving targeted delivery. Drug carriers achieve precise drug delivery through passive targeting capabilities (designing the particle size, surface charge, shape, etc. of the carrier) and active targeting functions (surface modification of the affinity group of the target, including antibodies, nucleic acid aptamers, peptides, small molecule aptamers, etc.) (Figure 2).
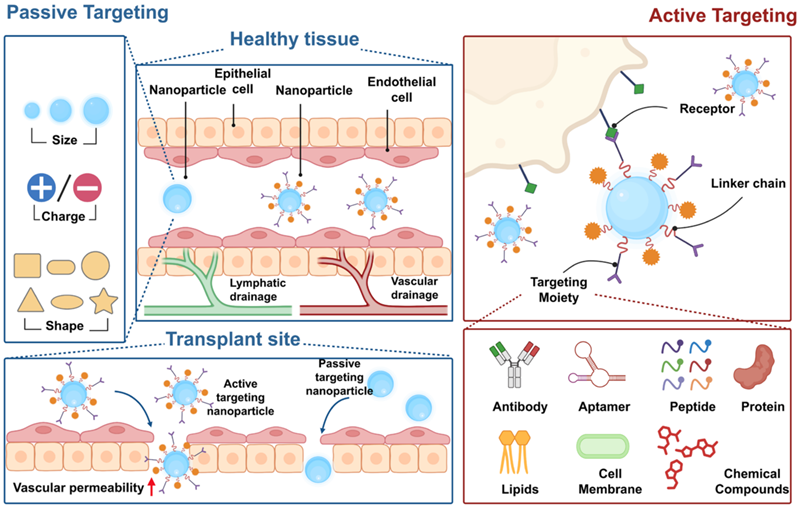
Figure 2, Passively and actively targeted drug delivery technologies in transplantation.
Although there are no anti-rejection nanomedicine formulations available for transplantation, scientists are still working tirelessly to seek a breakthrough in the clinical application of targeted delivery of anti-rejection drugs. The initiator of post-transplant rejection is the recipient's immune system, including multiple immune organs and the immune cell interactions and immune cascade reactions within them, and the sites of rejection are often located in the graft, immune organs and immune cells. Therefore, when designing drug delivery regimens, it is common to choose to deliver drugs to these specific targets in order to achieve more precise drug delivery and avoid off-target effects.
This review focuses on summarising preclinical studies of nanotechnology-based anti-rejection drug delivery. The main design concepts of delivery ISA are described in terms of immune organ targeting, graft targeting and immune cell targeting. It also analyses the difficulties and solutions of the targeted delivery system in clinical translation with respect to the specific targeting of organs or cells and the different characteristics of the nanomedicine delivery platforms. Finally, the review suggests that by designing actively targeted and environmentally responsive drugs and carriers, it is expected to achieve better postoperative anti-rejection effects and avoid systemic immunosuppression, and to improve the survival of grafts, and to reduce the chances of postoperative infections and tumour recurrence.
The team has published more than 100 papers in journals such as PNAS, Nature Communications, Cancer Res., Adv. Mater., ACS Nano, Biomaterials, and J. Controlled Release. In particular, in recent years, focusing on transplantation immunity as a major clinical problem, he has published several research papers in top journals including Am J Transplant, J Control Release, eBioMedicine, and Advanced Materials using molecular self-assembly technology in the targeted delivery of immunosuppressants and molecular imaging research. Research Papers. The research team is looking forward to advancing the clinical applications and translational research in transplantation through nanobiotechnology innovation based on supramolecular self-assembly.
This work was supported by the Jinan Microecological Biomedicine Shandong Laboratory (No. JNL-2022010B), the Major Basic Research Project of Natural Science Foundation of Shandong Province (No. ZR2023ZD59), and the National Natural Science Foundation of China (No. 82273490, 82073296, 81773193).